Decoding seasonal changes: soil parameters and microbial communities in tropical dry deciduous forests
- PMID: 38440136
- PMCID: PMC10910104
- DOI: 10.3389/fmicb.2024.1258934
Decoding seasonal changes: soil parameters and microbial communities in tropical dry deciduous forests
Abstract
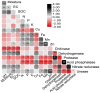
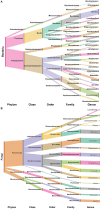
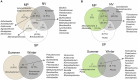
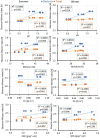
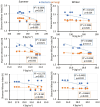
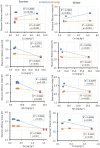
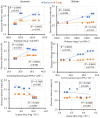
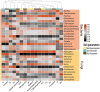
In dry deciduous tropical forests, both seasons (winter and summer) offer habitats that are essential ecologically. How these seasonal changes affect soil properties and microbial communities is not yet fully understood. This study aimed to investigate the influence of seasonal fluctuations on soil characteristics and microbial populations. The soil moisture content dramatically increases in the summer. However, the soil pH only gradually shifts from acidic to slightly neutral. During the summer, electrical conductivity (EC) values range from 0.62 to 1.03 ds m-1, in contrast to their decline in the winter. The levels of soil macronutrients and micronutrients increase during the summer, as does the quantity of soil organic carbon (SOC). A two-way ANOVA analysis reveals limited impacts of seasonal fluctuations and specific geographic locations on the amounts of accessible nitrogen (N) and phosphorus (P). Moreover, dehydrogenase, nitrate reductase, and urease activities rise in the summer, while chitinase, protease, and acid phosphatase activities are more pronounced in the winter. The soil microbes were identified in both seasons through 16S rRNA and ITS (Internal Transcribed Spacer) gene sequencing. Results revealed Proteobacteria and Ascomycota as predominant bacterial and fungal phyla. However, Bacillus, Pseudomonas, and Burkholderia are dominant bacterial genera, and Aspergillus, Alternaria, and Trichoderma are dominant fungal genera in the forest soil samples. Dominant bacterial and fungal genera may play a role in essential ecosystem services such as soil health management and nutrient cycling. In both seasons, clear relationships exist between soil properties, including pH, moisture, iron (Fe), zinc (Zn), and microbial diversity. Enzymatic activities and microbial shift relate positively with soil parameters. This study highlights robust soil-microbial interactions that persist mainly in the top layers of tropical dry deciduous forests in the summer and winter seasons. It provides insights into the responses of soil-microbial communities to seasonal changes, advancing our understanding of ecosystem dynamics and biodiversity preservation.
Keywords: bacteria; forest soil; fungi; seasonal variation; soil parameters.
Copyright © 2024 Solanki, Gurjar, Sharma, Wang, Kumar, Solanki, Kumar Divvela, Yadav and Kashyap.
Conflict of interest statement
PK was employed by Contec Global Agro Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Land Cover and Seasonal Variations Shape Soil Microbial Communities and Nutrient Cycling in Madagascar Tropical Forests.Microb Ecol. 2025 Jun 4;88(1):60. doi: 10.1007/s00248-025-02561-w. Microb Ecol. 2025. PMID: 40467851 Free PMC article.
-
Seasonal response of soil microbial community structure and life history strategies to winter snow cover change in a temperate forest.Sci Total Environ. 2024 Nov 1;949:175066. doi: 10.1016/j.scitotenv.2024.175066. Epub 2024 Jul 29. Sci Total Environ. 2024. PMID: 39079633
-
Space Rather than Seasonal Changes Explained More of the Spatiotemporal Variation of Tropical Soil Microbial Communities.Microbiol Spectr. 2022 Dec 21;10(6):e0184622. doi: 10.1128/spectrum.01846-22. Epub 2022 Nov 23. Microbiol Spectr. 2022. PMID: 36416607 Free PMC article.
-
Composition and Diversity of Soil Fungi in Dipterocarpaceae-Dominated Seasonal Tropical Forests in Thailand.Microbes Environ. 2018 Jul 4;33(2):135-143. doi: 10.1264/jsme2.ME17168. Epub 2018 May 30. Microbes Environ. 2018. PMID: 29848838 Free PMC article.
-
Forest Soil Bacteria: Diversity, Involvement in Ecosystem Processes, and Response to Global Change.Microbiol Mol Biol Rev. 2017 Apr 12;81(2):e00063-16. doi: 10.1128/MMBR.00063-16. Print 2017 Jun. Microbiol Mol Biol Rev. 2017. PMID: 28404790 Free PMC article. Review.
Cited by
-
Functional areas shape indoor microbial structure and potential risks in university dormitories.Front Microbiol. 2025 Jul 15;16:1604064. doi: 10.3389/fmicb.2025.1604064. eCollection 2025. Front Microbiol. 2025. PMID: 40735623 Free PMC article.
-
Subtle changes in topsoil microbial communities of drained forested peatlands after prolonged drought.Environ Microbiol Rep. 2024 Dec;16(6):e70041. doi: 10.1111/1758-2229.70041. Environ Microbiol Rep. 2024. PMID: 39512007 Free PMC article.
-
Land Cover and Seasonal Variations Shape Soil Microbial Communities and Nutrient Cycling in Madagascar Tropical Forests.Microb Ecol. 2025 Jun 4;88(1):60. doi: 10.1007/s00248-025-02561-w. Microb Ecol. 2025. PMID: 40467851 Free PMC article.
References
-
- Allard V., Robin C., Newton P. C. D., Lieffering M., Soussana J. F. (2006). Short and long-term effects of elevated CO2 on Lolium perenne rhizodeposition and its consequences on soil organic matter turnover and plant N yield. Soil Biol. Biochem. 38, 1178–1187. doi: 10.1016/J.SOILBIO.2005.10.002 - DOI
-
- Anderson L. J. (2011). Aboveground-belowground linkages: biotic interactions, ecosystem processes, and global change. EOS Trans. Am. Geophys. Union 92:222. doi: 10.1029/2011EO260011 - DOI
-
- Asigbaase M., Dawoe E., Sjogersten S., Lomax B. H. (2021). Decomposition and nutrient mineralisation of leaf litter in smallholder cocoa agroforests: a comparison of organic and conventional farms in Ghana. J. Soils Sediments 21, 1010–1023. doi: 10.1007/S11368-020-02844-4/FIGURES/6 - DOI
LinkOut - more resources
Full Text Sources

