Ligand effects, solvent cooperation, and large kinetic solvent deuterium isotope effects in gold(I)-catalyzed intramolecular alkene hydroamination
- PMID: 38440168
- PMCID: PMC10910400
- DOI: 10.3762/bjoc.20.43
Ligand effects, solvent cooperation, and large kinetic solvent deuterium isotope effects in gold(I)-catalyzed intramolecular alkene hydroamination
Abstract
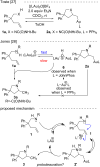
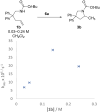
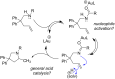
Kinetic studies on the intramolecular hydroamination of protected variants of 2,2-diphenylpent-4-en-1-amine were carried out under a variety of conditions with cationic gold catalysts supported by phosphine ligands. The impact of ligand on gold, protecting group on nitrogen, and solvent and additive on reaction rates was determined. The most effective reactions utilized more Lewis basic ureas, and more electron-withdrawing phosphines. A DCM/alcohol cooperative effect was quantified, and a continuum of isotope effects was measured with low KIE's in the absence of deuterated alcoholic solvent, increasing to large solvent KIE's when comparing reactions in pure MeOH to those in pure MeOH-d4. The effects are interpreted both within the context of a classic gold π-activation/protodeauration mechanism and a general acid-catalyzed mechanism without intermediate gold alkyls.
Keywords: alkene hydroamination; general acid catalysis; gold catalysis; isotope effect; phosphine ligand effect; solvent effect.
Copyright © 2024, Lan et al.
Figures







References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
