Switchable molecular tweezers: design and applications
- PMID: 38440175
- PMCID: PMC10910529
- DOI: 10.3762/bjoc.20.45
Switchable molecular tweezers: design and applications
Abstract
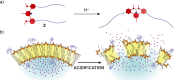
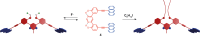
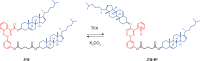
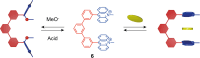
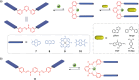
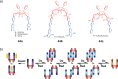
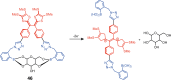
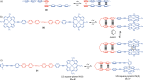
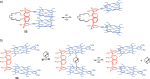
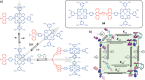
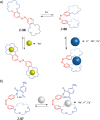
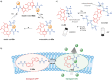
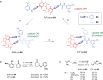
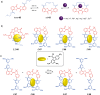
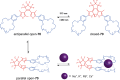
Switchable molecular tweezers are a unique class of molecular switches that, like their macroscopic analogs, exhibit mechanical motion between an open and closed conformation in response to stimuli. Such systems constitute an essential component of artificial molecular machines. This review will present selected examples of switchable molecular tweezers and their potential applications. The first part will be devoted to chemically responsive tweezers, including stimuli such as pH, metal coordination, and anion binding. Then, redox-active and photochemical tweezers will be presented.
Keywords: coordination; molecular recognition; molecular switches; photoswitch; redox; supramolecular chemistry.
Copyright © 2024, Msellem et al.
Figures

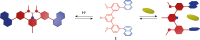
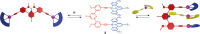
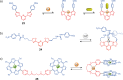
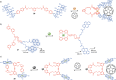
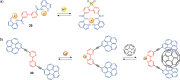
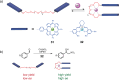
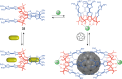
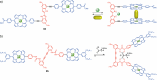

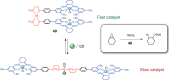
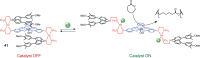

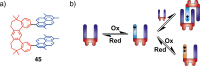
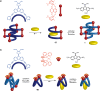

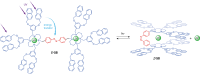
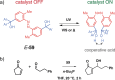
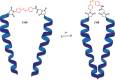
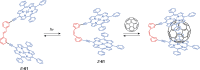
References
Publication types
LinkOut - more resources
Full Text Sources
