The dark side of stemness - the role of hematopoietic stem cells in development of blood malignancies
- PMID: 38440231
- PMCID: PMC10910019
- DOI: 10.3389/fonc.2024.1308709
The dark side of stemness - the role of hematopoietic stem cells in development of blood malignancies
Erratum in
-
Correction: The dark side of stemness - the role of hematopoietic stem cells in development of blood malignancies.Front Oncol. 2025 Jun 24;15:1633832. doi: 10.3389/fonc.2025.1633832. eCollection 2025. Front Oncol. 2025. PMID: 40630204 Free PMC article.
Abstract
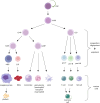
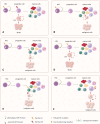
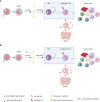
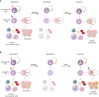
Hematopoietic stem cells (HSCs) produce all blood cells throughout the life of the organism. However, the high self-renewal and longevity of HSCs predispose them to accumulate mutations. The acquired mutations drive preleukemic clonal hematopoiesis, which is frequent among elderly people. The preleukemic state, although often asymptomatic, increases the risk of blood cancers. Nevertheless, the direct role of preleukemic HSCs is well-evidenced in adult myeloid leukemia (AML), while their contribution to other hematopoietic malignancies remains less understood. Here, we review the evidence supporting the role of preleukemic HSCs in different types of blood cancers, as well as present the alternative models of malignant evolution. Finally, we discuss the clinical importance of preleukemic HSCs in choosing the therapeutic strategies and provide the perspective on further studies on biology of preleukemic HSCs.
Keywords: acute lymphoblastic leukemia; acute myeloid leukemia; chronic lymphocytic leukemia; chronic myeloid leukemia; clonal hematopoiesis; hematopoietic stem cell; mature cell neoplasm; preleukemic state.
Copyright © 2024 Filipek-Gorzała, Kwiecińska, Szade and Szade.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Hoffman R, Benz EJ, Silberstein LE, Heslop HE, Weitz JI, Anastasi J, et al. Hematology: basic principles and practice. (2018).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

