Controlled-release hydrogel loaded with magnesium-based nanoflowers synergize immunomodulation and cartilage regeneration in tendon-bone healing
- PMID: 38440323
- PMCID: PMC10909705
- DOI: 10.1016/j.bioactmat.2024.02.024
Controlled-release hydrogel loaded with magnesium-based nanoflowers synergize immunomodulation and cartilage regeneration in tendon-bone healing
Abstract
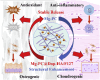
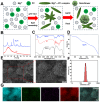
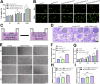
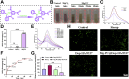
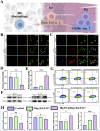
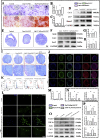
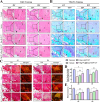
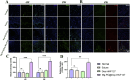
Tendon-bone interface injuries pose a significant challenge in tissue regeneration, necessitating innovative approaches. Hydrogels with integrated supportive features and controlled release of therapeutic agents have emerged as promising candidates for the treatment of such injuries. In this study, we aimed to develop a temperature-sensitive composite hydrogel capable of providing sustained release of magnesium ions (Mg2+). We synthesized magnesium-Procyanidin coordinated metal polyphenol nanoparticles (Mg-PC) through a self-assembly process and integrated them into a two-component hydrogel. The hydrogel was composed of dopamine-modified hyaluronic acid (Dop-HA) and F127. To ensure controlled release and mitigate the "burst release" effect of Mg2+, we covalently crosslinked the Mg-PC nanoparticles through coordination bonds with the catechol moiety within the hydrogel. This crosslinking strategy extended the release window of Mg2+ concentrations for up to 56 days. The resulting hydrogel (Mg-PC@Dop-HA/F127) exhibited favorable properties, including injectability, thermosensitivity and shape adaptability, making it suitable for injection and adaptation to irregularly shaped supraspinatus implantation sites. Furthermore, the hydrogel sustained the release of Mg2+ and Procyanidins, which attracted mesenchymal stem and progenitor cells, alleviated inflammation, and promoted macrophage polarization towards the M2 phenotype. Additionally, it enhanced collagen synthesis and mineralization, facilitating the repair of the tendon-bone interface. By incorporating multilevel metal phenolic networks (MPN) to control ion release, these hybridized hydrogels can be customized for various biomedical applications.
Keywords: Controlled release; Immunomodulation; Magnesium; Metal–phenolic networks; Self-assembly process; Tendon-bone interface.
© 2024 The Authors.
Conflict of interest statement
The authors declared that they have no conflicts of interest to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Figures




References
-
- Atesok K., Doral M.N., Karlsson J., Egol K.A., Jazrawi L.M., Coelho P.G., Martinez A., Matsumoto T., Owens B.D., Ochi M., Hurwitz S.R., Atala A., Fu F.H., Lu H.H., Rodeo S.A. Multilayer scaffolds in orthopaedic tissue engineering. Knee Surg. Sports Traumatol. Arthrosc. J. ESSKA. 2016;24(7):2365–2373. doi: 10.1007/s00167-014-3453-z. - DOI - PubMed
LinkOut - more resources
Full Text Sources

