Bioinspired engineering ADSC nanovesicles thermosensitive hydrogel enhance autophagy of dermal papilla cells for androgenetic alopecia treatment
- PMID: 38440324
- PMCID: PMC10911949
- DOI: 10.1016/j.bioactmat.2024.02.023
Bioinspired engineering ADSC nanovesicles thermosensitive hydrogel enhance autophagy of dermal papilla cells for androgenetic alopecia treatment
Abstract
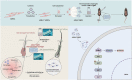
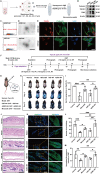
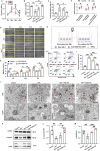
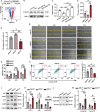
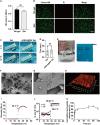
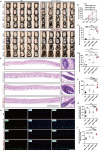
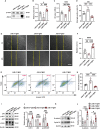
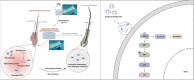
Androgenic alopecia (AGA) is a highly prevalent form of non-scarring alopecia but lacks effective treatments. Stem cell exosomes have similar repair effects to stem cells, suffer from the drawbacks of high cost and low yield yet. Cell-derived nanovesicles acquired through mechanical extrusion exhibit favorable biomimetic properties similar to exosomes, enabling them to efficiently encapsulate substantial quantities of therapeutic proteins. In this study, we observed that JAM-A, an adhesion protein, resulted in a significantly increased the adhesion and resilience of dermal papilla cells to form snap structures against damage caused by dihydrotestosterone and macrophages, thereby facilitating the process of hair regrowth in cases of AGA. Consequently, adipose-derived stem cells were modified to overexpress JAM-A to produce engineered JAM-A overexpressing nanovesicles (JAM-AOE@NV). The incorporation of JAM-AOE@NV into a thermosensitive hydrogel matrix (JAM-AOE@NV Gel) to effectively addresses the limitations associated with the short half-life of JAM-AOE@NV, and resulted in the achievement of a sustained-release profile for JAM-AOE@NV. The physicochemical characteristics of the JAM-AOE@NV Gel were analyzed and assessed for its efficacy in promoting hair regrowth in vivo and vitro. The JAM-AOE@NV Gel, thus, presents a novel therapeutic approach and theoretical framework for promoting the treatment of low cell adhesion diseases similar to AGA.
Keywords: Androgenic alopecia; Autophagy; Hydrogel; JAM-A; Nanovesicles.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing financial interests or personal relationships that could have influenced the work reported in this paper.
Figures
Similar articles
-
Adipose Mesenchymal Stromal Cell-Derived Exosomes Carrying MiR-122-5p Antagonize the Inhibitory Effect of Dihydrotestosterone on Hair Follicles by Targeting the TGF-β1/SMAD3 Signaling Pathway.Int J Mol Sci. 2023 Mar 16;24(6):5703. doi: 10.3390/ijms24065703. Int J Mol Sci. 2023. PMID: 36982775 Free PMC article.
-
Potential of mesenchymal stem cell-derived exosomes in the treatment of androgenetic alopecia.World J Stem Cells. 2025 May 26;17(5):107078. doi: 10.4252/wjsc.v17.i5.107078. World J Stem Cells. 2025. PMID: 40503362 Free PMC article.
-
Adipose-Derived Stem Cell Exosomes Antagonize the Inhibitory Effect of Dihydrotestosterone on Hair Follicle Growth by Activating Wnt/β-Catenin Pathway.Stem Cells Int. 2023 Sep 27;2023:5548112. doi: 10.1155/2023/5548112. eCollection 2023. Stem Cells Int. 2023. PMID: 37810630 Free PMC article.
-
Regenerative medicine strategies for hair growth and regeneration: A narrative review of literature.Regen Ther. 2022 Oct 31;21:527-539. doi: 10.1016/j.reth.2022.10.005. eCollection 2022 Dec. Regen Ther. 2022. PMID: 36382136 Free PMC article. Review.
-
Molecular basis of androgenetic alopecia: From androgen to paracrine mediators through dermal papilla.J Dermatol Sci. 2011 Jan;61(1):1-6. doi: 10.1016/j.jdermsci.2010.10.015. Epub 2010 Nov 3. J Dermatol Sci. 2011. PMID: 21167691 Review.
Cited by
-
Inflammatory memory-activated biomimetic nanovesicles regulate neutrophil plasticity and metabolic reprogramming for rapid diabetic wound healing via targeting miR-193a-5p/TLR4/JNK/P38 MAPK pathways.J Nanobiotechnology. 2025 Feb 17;23(1):115. doi: 10.1186/s12951-025-03193-5. J Nanobiotechnology. 2025. PMID: 39962468 Free PMC article.
-
Mechanisms and clinical progress of adipose-derived stem cells and their derivatives in the treatment of hair loss.Stem Cell Res Ther. 2025 Aug 8;16(1):439. doi: 10.1186/s13287-025-04560-7. Stem Cell Res Ther. 2025. PMID: 40781338 Free PMC article. Review.
-
Autophagy Dysfunction: The Kernel of Hair Loss?Clin Cosmet Investig Dermatol. 2024 May 20;17:1165-1181. doi: 10.2147/CCID.S462294. eCollection 2024. Clin Cosmet Investig Dermatol. 2024. PMID: 38800357 Free PMC article. Review.
-
Tremella polysaccharide microneedles loaded with magnetic dental pulp stem cell intracellular vesicles used for androgenic alopecia.Stem Cell Res Ther. 2025 Mar 31;16(1):161. doi: 10.1186/s13287-025-04219-3. Stem Cell Res Ther. 2025. PMID: 40165226 Free PMC article.
-
Extracellular vesicles and their mimetics: clinical application prospects in medical aesthetics.Burns Trauma. 2025 May 17;13:tkaf033. doi: 10.1093/burnst/tkaf033. eCollection 2025. Burns Trauma. 2025. PMID: 40757165 Free PMC article.
References
-
- Yang G., Chen Q., Wen D., Chen Z., Wang J., Chen G., et al. A therapeutic microneedle patch made from hair-derived keratin for promoting hair regrowth. ACS Nano. 2019;13:4354–4360. - PubMed
-
- Manabe M., Tsuboi R., Itami S., Osada S.I., Amoh Y., Ito T., et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version. J. Dermatol. 2018;45:1031–1043. - PubMed
-
- Jimenez F., Alam M., Vogel J.E., Avram M. Hair transplantation: basic overview. J. Am. Acad. Dermatol. 2021;85:803–814. - PubMed
-
- Gupta A.K., Venkataraman M., Talukder M., Bamimore M.A. Finasteride for hair loss: a review. J. Dermatol. Treat. 2022;33:1938–1946. - PubMed
-
- Goren A., Naccarato T. Minoxidil in the treatment of androgenetic alopecia. Dermatol. Ther. 2018;31 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

