Fabrication and properties of PLA/β-TCP scaffolds using liquid crystal display (LCD) photocuring 3D printing for bone tissue engineering
- PMID: 38440328
- PMCID: PMC10910430
- DOI: 10.3389/fbioe.2024.1273541
Fabrication and properties of PLA/β-TCP scaffolds using liquid crystal display (LCD) photocuring 3D printing for bone tissue engineering
Abstract
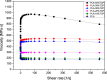
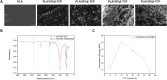
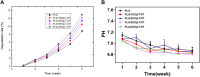
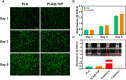
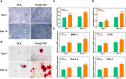
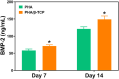
Introduction: Bone defects remain a thorny challenge that clinicians have to face. At present, scaffolds prepared by 3D printing are increasingly used in the field of bone tissue repair. Polylactic acid (PLA) has good thermoplasticity, processability, biocompatibility, and biodegradability, but the PLA is brittle and has poor osteogenic performance. Beta-tricalcium phosphate (β-TCP) has good mechanical properties and osteogenic induction properties, which can make up for the drawbacks of PLA. Methods: In this study, photocurable biodegradable polylactic acid (bio-PLA) was utilized as the raw material to prepare PLA/β-TCP slurries with varying β-TCP contents (β-TCP dosage at 0%, 10%, 20%, 30%, 35% of the PLA dosage, respectively). The PLA/β-TCP scaffolds were fabricated using liquid crystal display (LCD) light-curing 3D printing technology. The characterization of the scaffolds was assessed, and the biological activity of the scaffold with the optimal compressive strength was evaluated. The biocompatibility of the scaffold was assessed through CCK-8 assays, hemocompatibility assay and live-dead staining experiments. The osteogenic differentiation capacity of the scaffold on MC3T3-E1 cells was evaluated through alizarin red staining, alkaline phosphatase (ALP) detection, immunofluorescence experiments, and RT-qPCR assays. Results: The prepared scaffold possesses a three-dimensional network structure, and with an increase in the quantity of β-TCP, more β-TCP particles adhere to the scaffold surface. The compressive strength of PLA/β-TCP scaffolds exhibits a trend of initial increase followed by decrease with an increasing amount of β-TCP, reaching a maximum value of 52.1 MPa at a 10% β-TCP content. Degradation rate curve results indicate that with the passage of time, the degradation rate of the scaffold gradually increases, and the pH of the scaffold during degradation shows an alkaline tendency. Additionally, Live/dead staining and blood compatibility experiments suggest that the prepared PLA/β-TCP scaffold demonstrates excellent biocompatibility. CCK-8 experiments indicate that the PLA/β-TCP group promotes cell proliferation, and the prepared PLA/β-TCP scaffold exhibits a significant ability to enhance the osteogenic differentiation of MC3T3-E1 cells in vitro. Discussion: 3D printed LCD photocuring PLA/β-TCP scaffolds could improve surface bioactivity and lead to better osteogenesis, which may provide a unique strategy for developing bioactive implants in orthopedic applications.
Keywords: 3D printed scaffolds; beta-tricalcium phosphate; bone tissue engineering; liquid crystal display; polylactic acid.
Copyright © 2024 Wang, Ye, Chen, Zhang, Zeng, Liu, Tan and Jie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
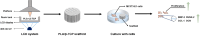


References
-
- Canciani E., Straticò P., Varasano S., Dellavia C., Sciarrini C., Petrizzi L., et al. (2023). Polylevolysine and fibronectin-loaded nano-hydroxyapatite/PGLA/dextran-based scaffolds for improving bone regeneration: a histomorphometric in animal study. Int. J. Mol. Sci. 24 (9), 8137. 10.3390/ijms24098137 - DOI - PMC - PubMed
-
- da Rocha L. B. N., Sousa R. B., Dos Santos M. V. B., Argolo Neto N. M., Soares L. L. d. S., Alves F. L. C., et al. (2023). Development of a new biomaterial based on cashew tree gum (Anarcadium occidentale L.) enriched with hydroxyapatite and evaluation of cytotoxicity in adipose-derived stem cell cultures. Int. J. Biol. Macromol. 242 (Pt 2), 124864. 10.1016/j.ijbiomac.2023.124864 - DOI - PubMed
LinkOut - more resources
Full Text Sources

