Research gaps and future needs for allergen prediction in food safety
- PMID: 38440401
- PMCID: PMC10911423
- DOI: 10.3389/falgy.2024.1297547
Research gaps and future needs for allergen prediction in food safety
Abstract
The allergenicity and protein risk assessments in food safety are facing new challenges. Demands for healthier and more sustainable food systems have led to significant advances in biotechnology, the development of more complex foods, and the search for alternative protein sources. All this has increased the pressure on the safety assessment prediction approaches anchored into requirements defined in the late 90's. In 2022, the EFSA's Panel on Genetically Modified Organisms published a scientific opinion focusing on the developments needed for allergenicity and protein safety assessments of new products derived from biotechnology. Here, we further elaborate on the main elements described in this scientific opinion and prioritize those development needs requiring critical attention. The starting point of any new recommendation would require a focus on clinical relevance and the development of a fit-for-purpose database targeted for specific risk assessment goals. Furthermore, it is imperative to review and clarify the main purpose of the allergenicity risk assessment. An internationally agreed consensus on the overall purpose of allergenicity risk assessment will accelerate the development of fit-for-purpose methodologies, where the role of exposure should be better clarified. Considering the experience gained over the last 25 years and recent scientific developments in the fields of biotechnology, allergy, and risk assessment, it is time to revise and improve the allergenicity safety assessment to ensure the reliability of allergenicity assessments for food of the future.
Keywords: allergy; bioinformatics; elicitation; food allergy; predictive; protein safety; risk assessment; sensitisation.
© 2024 Fernandez, Danisman, Taheri Boroujerdi, Kazemi, Moreno and Epstein.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
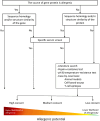
References
-
- ISAAA. Available online at: https://www.isaaa.org/gmapprovaldatabase/ (accessed July 15, 2023).
-
- European Food Safety Authority—EFSA. Available online at: https://www.efsa.europa.eu/en/publications (accessed July 15, 2023).
-
- European Commission—EC. Available online at: https://webgate.ec.europa.eu/dyna2/gm-register/ (accessed July 15, 2023).
-
- European Commission—EC. Available online at: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/eu... (accessed July 15, 2023).
-
- Devos Y, Arena M, Ashe S, Blanck M, Bray E, Broglis A, et al. Addressing the need for safe, nutritious and sustainable food: outcomes of the “ONE—health, environment & society—conference 2022”. Trends Food Sci Technol. (2022) 129:164–78. 10.1016/j.tifs.2022.09.014 - DOI
Publication types
LinkOut - more resources
Full Text Sources

