CD274 (PD-L1) negatively regulates M1 macrophage polarization in ALI/ARDS
- PMID: 38440722
- PMCID: PMC10909908
- DOI: 10.3389/fimmu.2024.1344805
CD274 (PD-L1) negatively regulates M1 macrophage polarization in ALI/ARDS
Abstract
Background: Acute lung injury (ALI)/severe acute respiratory distress syndrome (ARDS) is a serious clinical syndrome characterized by a high mortality rate. The pathophysiological mechanisms underlying ALI/ARDS remain incompletely understood. Considering the crucial role of immune infiltration and macrophage polarization in the pathogenesis of ALI/ARDS, this study aims to identify key genes associated with both ALI/ARDS and M1 macrophage polarization, employing a combination of bioinformatics and experimental approaches. The findings could potentially reveal novel biomarkers for the diagnosis and management of ALI/ARDS.
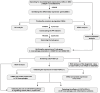
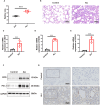
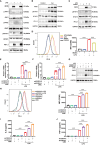
Methods: Gene expression profiles relevant to ALI were retrieved from the GEO database to identify co-upregulated differentially expressed genes (DEGs). GO and KEGG analyses facilitated functional annotation and pathway elucidation. PPI networks were constructed to identify hub genes, and differences in immune cell infiltration were subsequently examined. The expression of hub genes in M1 versus M2 macrophages was evaluated using macrophage polarization datasets. The diagnostic utility of CD274 (PD-L1) for ARDS was assessed by receiver operating characteristic (ROC) analysis in a validation dataset. Experimental confirmation was conducted using two LPS-induced M1 macrophage models and an ALI mouse model. The role of CD274 (PD-L1) in M1 macrophage polarization and associated proinflammatory cytokine production was further investigated by siRNA-mediated silencing.
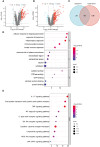
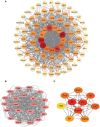
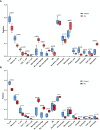
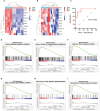
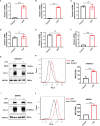
Results: A total of 99 co-upregulated DEGs were identified in two ALI-linked datasets. Enrichment analysis revealed that these DEGs were mainly involved in immune-inflammatory pathways. The following top 10 hub genes were identified from the PPI network: IL-6, IL-1β, CXCL10, CD274, CCL2, TLR2, CXCL1, CCL3, IFIT1, and IFIT3. Immune infiltration analysis revealed a significantly increased abundance of M1 and M2 macrophages in lung tissue from the ALI group compared to the control group. Subsequent analysis confirmed that CD274 (PD-L1), a key immunological checkpoint molecule, was highly expressed within M1 macrophages. ROC analysis validated CD274 (PD-L1) as a promising biomarker for the diagnosis of ARDS. Both in vitro and in vivo experiments supported the bioinformatics analysis and confirmed that the JAK-STAT3 pathway promotes CD274 (PD-L1) expression on M1 macrophages. Importantly, knockdown of CD274 (PD-L1) expression potentiated M1 macrophage polarization and enhanced proinflammatory cytokines production.
Conclusion: This study demonstrates a significant correlation between CD274 (PD-L1) and M1 macrophages in ALI/ARDS. CD274 (PD-L1) functions as a negative regulator of M1 polarization and the secretion of proinflammatory cytokines in macrophages. These findings suggest potential new targets for the diagnosis and treatment of ALI/ARDS.
Keywords: M1 polarization; PD-L1; biomarker; immune infiltration; inflammation; macrophage; severe acute respiratory distress syndrome; stat3.
Copyright © 2024 Tang, Yang, Xie, Yang, Wang, Li, Liu and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
IFIH1/IRF1/STAT1 promotes sepsis associated inflammatory lung injury via activating macrophage M1 polarization.Int Immunopharmacol. 2023 Jan;114:109478. doi: 10.1016/j.intimp.2022.109478. Epub 2022 Nov 30. Int Immunopharmacol. 2023. PMID: 36462334 Free PMC article.
-
Human Umbilical Cord Mesenchymal Stem Cells Promote Macrophage PD-L1 Expression and Attenuate Acute Lung Injury in Mice.Curr Stem Cell Res Ther. 2022;17(6):564-575. doi: 10.2174/1574888X17666220127110332. Curr Stem Cell Res Ther. 2022. PMID: 35086457
-
Role of cuproptosis in mediating the severity of experimental malaria-associated acute lung injury/acute respiratory distress syndrome.Parasit Vectors. 2024 Oct 19;17(1):433. doi: 10.1186/s13071-024-06520-1. Parasit Vectors. 2024. PMID: 39427197 Free PMC article.
-
Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome.Inflamm Res. 2020 Sep;69(9):883-895. doi: 10.1007/s00011-020-01378-2. Epub 2020 Jul 10. Inflamm Res. 2020. PMID: 32647933 Free PMC article. Review.
-
Natural Compounds Regulate Macrophage Polarization and Alleviate Inflammation Against ALI/ARDS.Biomolecules. 2025 Jan 29;15(2):192. doi: 10.3390/biom15020192. Biomolecules. 2025. PMID: 40001495 Free PMC article. Review.
Cited by
-
Advancements in omics technologies: Molecular mechanisms of acute lung injury and acute respiratory distress syndrome (Review).Int J Mol Med. 2025 Mar;55(3):38. doi: 10.3892/ijmm.2024.5479. Epub 2025 Jan 3. Int J Mol Med. 2025. PMID: 39749711 Free PMC article. Review.
-
Exosome Secretion Regulation in Mice with Acute Lung Injury.J Inflamm Res. 2025 Jun 3;18:7151-7165. doi: 10.2147/JIR.S506693. eCollection 2025. J Inflamm Res. 2025. PMID: 40487284 Free PMC article.
-
Hsa-miR-194-5p regulates TRAF6-mediated M1 macrophage apoptosis in recurrent spontaneous abortion.J Mol Histol. 2025 May 26;56(3):166. doi: 10.1007/s10735-025-10464-w. J Mol Histol. 2025. PMID: 40418413
-
Aberrant Expression of SLC7A11 Impairs the Antimicrobial Activities of Macrophages in Staphylococcus Aureus Osteomyelitis in Mice.Int J Biol Sci. 2024 Apr 22;20(7):2555-2575. doi: 10.7150/ijbs.93592. eCollection 2024. Int J Biol Sci. 2024. PMID: 38725861 Free PMC article.
-
IFIT3: a crucial mediator in innate immunity and tumor progression with therapeutic implications.Front Immunol. 2025 Feb 24;16:1515718. doi: 10.3389/fimmu.2025.1515718. eCollection 2025. Front Immunol. 2025. PMID: 40061935 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

