Key considerations to improve the normalization, interpretation and reproducibility of morbidity data in mammalian models of viral disease
- PMID: 38440823
- PMCID: PMC10941659
- DOI: 10.1242/dmm.050511
Key considerations to improve the normalization, interpretation and reproducibility of morbidity data in mammalian models of viral disease
Abstract
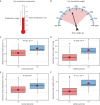
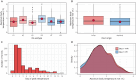
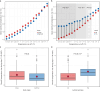
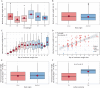
Viral pathogenesis and therapeutic screening studies that utilize small mammalian models rely on the accurate quantification and interpretation of morbidity measurements, such as weight and body temperature, which can vary depending on the model, agent and/or experimental design used. As a result, morbidity-related data are frequently normalized within and across screening studies to aid with their interpretation. However, such data normalization can be performed in a variety of ways, leading to differences in conclusions drawn and making comparisons between studies challenging. Here, we discuss variability in the normalization, interpretation, and presentation of morbidity measurements for four model species frequently used to study a diverse range of human viral pathogens - mice, hamsters, guinea pigs and ferrets. We also analyze findings aggregated from influenza A virus-infected ferrets to contextualize this discussion. We focus on serially collected weight and temperature data to illustrate how the conclusions drawn from this information can vary depending on how raw data are collected, normalized and measured. Taken together, this work supports continued efforts in understanding how normalization affects the interpretation of morbidity data and highlights best practices to improve the interpretation and utility of these findings for extrapolation to public health contexts.
Keywords: Ferret; Pathogenesis; Virus.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

Similar articles
-
Utility of Human In Vitro Data in Risk Assessments of Influenza A Virus Using the Ferret Model.J Virol. 2023 Jan 31;97(1):e0153622. doi: 10.1128/jvi.01536-22. Epub 2023 Jan 5. J Virol. 2023. PMID: 36602361 Free PMC article.
-
Pathogenesis, Humoral Immune Responses, and Transmission between Cohoused Animals in a Ferret Model of Human Respiratory Syncytial Virus Infection.J Virol. 2018 Jan 30;92(4):e01322-17. doi: 10.1128/JVI.01322-17. Print 2018 Feb 15. J Virol. 2018. PMID: 29187546 Free PMC article.
-
Experimental models for study of common respiratory viruses.Environ Health Perspect. 1980 Apr;35:107-12. doi: 10.1289/ehp.8035107. Environ Health Perspect. 1980. PMID: 6250807 Free PMC article.
-
Ferrets as Models for Viral Respiratory Disease.Comp Med. 2023 Jun 1;73(3):187-193. doi: 10.30802/AALAS-CM-22-000064. Epub 2023 May 31. Comp Med. 2023. PMID: 37258084 Free PMC article. Review.
-
Animal models for influenza virus pathogenesis, transmission, and immunology.J Immunol Methods. 2014 Aug;410:60-79. doi: 10.1016/j.jim.2014.03.023. Epub 2014 Apr 4. J Immunol Methods. 2014. PMID: 24709389 Free PMC article. Review.
Cited by
-
Eleven quick tips to unlock the power of in vivo data science.PLoS Comput Biol. 2025 Apr 17;21(4):e1012947. doi: 10.1371/journal.pcbi.1012947. eCollection 2025 Apr. PLoS Comput Biol. 2025. PMID: 40245007 Free PMC article. No abstract available.
-
Machine learning approaches for influenza A virus risk assessment identifies predictive correlates using ferret model in vivo data.Commun Biol. 2024 Aug 1;7(1):927. doi: 10.1038/s42003-024-06629-0. Commun Biol. 2024. PMID: 39090358 Free PMC article.
-
Data alchemy, from lab to insight: Transforming in vivo experiments into data science gold.PLoS Pathog. 2024 Aug 29;20(8):e1012460. doi: 10.1371/journal.ppat.1012460. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39208339 Free PMC article. No abstract available.
-
An aggregated dataset of serial morbidity and titer measurements from influenza A virus-infected ferrets.Sci Data. 2024 May 17;11(1):510. doi: 10.1038/s41597-024-03256-6. Sci Data. 2024. PMID: 38760422 Free PMC article.
References
-
- Atkins, C., Miao, J., Kalveram, B., Juelich, T., Smith, J. K., Perez, D., Zhang, L., Westover, J. L. B., Van Wettere, A. J., Gowen, B. B.et al. (2018). Natural history and pathogenesis of wild-type Marburg virus infection in STAT2 knockout hamsters. J. Infect. Dis. 218, S438-S447. 10.1093/infdis/jiy457 - DOI - PMC - PubMed
-
- Balls, M. and Straughan, D. W. (1996). The three Rs of Russell & Burch and the testing of biological products. Dev. Biol. Stand. 86, 11-18. - PubMed
-
- Belser, J. A., Johnson, A., Pulit-Penaloza, J. A., Pappas, C., Pearce, M. B., Tzeng, W. P., Hossain, M. J., Ridenour, C., Wang, L., Chen, L. M.et al. (2017). Pathogenicity testing of influenza candidate vaccine viruses in the ferret model. Virology 511, 135-141. 10.1016/j.virol.2017.08.024 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

