P. aeruginosa tRNA-fMet halves secreted in outer membrane vesicles suppress lung inflammation in cystic fibrosis
- PMID: 38440830
- PMCID: PMC11380944
- DOI: 10.1152/ajplung.00018.2024
P. aeruginosa tRNA-fMet halves secreted in outer membrane vesicles suppress lung inflammation in cystic fibrosis
Abstract
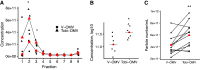
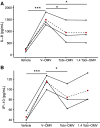
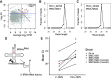
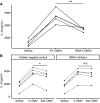
Although tobramycin increases lung function in people with cystic fibrosis (pwCF), the density of Pseudomonas aeruginosa (P. aeruginosa) in the lungs is only modestly reduced by tobramycin; hence, the mechanism whereby tobramycin improves lung function is not completely understood. Here, we demonstrate that tobramycin increases 5' tRNA-fMet halves in outer membrane vesicles (OMVs) secreted by laboratory and CF clinical isolates of P. aeruginosa. The 5' tRNA-fMet halves are transferred from OMVs into primary CF human bronchial epithelial cells (CF-HBEC), decreasing OMV-induced IL-8 and IP-10 secretion. In mouse lungs, increased expression of the 5' tRNA-fMet halves in OMVs attenuated KC (murine homolog of IL-8) secretion and neutrophil recruitment. Furthermore, there was less IL-8 and neutrophils in bronchoalveolar lavage fluid isolated from pwCF during the period of exposure to tobramycin versus the period off tobramycin. In conclusion, we have shown in mice and in vitro studies on CF-HBEC that tobramycin reduces inflammation by increasing 5' tRNA-fMet halves in OMVs that are delivered to CF-HBEC and reduce IL-8 and neutrophilic airway inflammation. This effect is predicted to improve lung function in pwCF receiving tobramycin for P. aeruginosa infection.NEW & NOTEWORTHY The experiments in this report identify a novel mechanism, whereby tobramycin reduces inflammation in two models of CF. Tobramycin increased the secretion of tRNA-fMet halves in OMVs secreted by P. aeruginosa, which reduced the OMV-LPS-induced inflammatory response in primary cultures of CF-HBEC and in mouse lung, an effect predicted to reduce lung damage in pwCF.
Keywords: P. aeruginosa; cystic fibrosis; host-pathogen; outer membrane vesicles; tRNA.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




Update of
-
P. aeruginosa tRNA-fMet halves secreted in outer membrane vesicles suppress lung inflammation in Cystic Fibrosis.bioRxiv [Preprint]. 2024 Feb 3:2024.02.03.578737. doi: 10.1101/2024.02.03.578737. bioRxiv. 2024. Update in: Am J Physiol Lung Cell Mol Physiol. 2024 May 1;326(5):L574-L588. doi: 10.1152/ajplung.00018.2024. PMID: 38352468 Free PMC article. Updated. Preprint.
Similar articles
-
P. aeruginosa tRNA-fMet halves secreted in outer membrane vesicles suppress lung inflammation in Cystic Fibrosis.bioRxiv [Preprint]. 2024 Feb 3:2024.02.03.578737. doi: 10.1101/2024.02.03.578737. bioRxiv. 2024. Update in: Am J Physiol Lung Cell Mol Physiol. 2024 May 1;326(5):L574-L588. doi: 10.1152/ajplung.00018.2024. PMID: 38352468 Free PMC article. Updated. Preprint.
-
Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD004197. doi: 10.1002/14651858.CD004197.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Jun 2;6:CD004197. doi: 10.1002/14651858.CD004197.pub6. PMID: 28440853 Free PMC article. Updated.
-
Colistimethate sodium powder and tobramycin powder for inhalation for the treatment of chronic Pseudomonas aeruginosa lung infection in cystic fibrosis: systematic review and economic model.Health Technol Assess. 2013 Dec;17(56):v-xvii, 1-181. doi: 10.3310/hta17560. Health Technol Assess. 2013. PMID: 24290164 Free PMC article.
-
Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis.Cochrane Database Syst Rev. 2014 Nov 10;(11):CD004197. doi: 10.1002/14651858.CD004197.pub4. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2017 Apr 25;4:CD004197. doi: 10.1002/14651858.CD004197.pub5. PMID: 25383937 Updated.
-
Pseudomonas aeruginosa Lipid A Structural Variants Induce Altered Immune Responses.Am J Respir Cell Mol Biol. 2024 Aug;71(2):207-218. doi: 10.1165/rcmb.2024-0059OC. Am J Respir Cell Mol Biol. 2024. PMID: 38656811 Free PMC article.
Cited by
-
Gene expression responses of CF airway epithelial cells exposed to elexacaftor/tezacaftor/ivacaftor suggest benefits beyond improved CFTR channel function.Am J Physiol Lung Cell Mol Physiol. 2024 Dec 1;327(6):L905-L916. doi: 10.1152/ajplung.00272.2024. Epub 2024 Oct 22. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 39437760 Free PMC article.
-
Let-7b-5p loaded Mesenchymal Stromal Cell Extracellular Vesicles reduce Pseudomonas-biofilm formation and inflammation in CF Bronchial Epithelial Cells.bioRxiv [Preprint]. 2025 May 28:2025.05.28.656674. doi: 10.1101/2025.05.28.656674. bioRxiv. 2025. PMID: 40501816 Free PMC article. Preprint.
-
Pseudomonas aeruginosa-derived extracellular vesicles enhance macrophage aerobic glycolysis that fuels inflammation.Front Microbiol. 2025 Jul 17;16:1619101. doi: 10.3389/fmicb.2025.1619101. eCollection 2025. Front Microbiol. 2025. PMID: 40746325 Free PMC article.
-
[Isolation and proteomic analysis of bacterial outer membrane vesicle subpopulations].Se Pu. 2025 May;43(5):529-538. doi: 10.3724/SP.J.1123.2024.10028. Se Pu. 2025. PMID: 40331616 Free PMC article. Chinese.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

