Human otic progenitor cell models of congenital hearing loss reveal potential pathophysiologic mechanisms of Zika virus and cytomegalovirus infections
- PMID: 38440980
- PMCID: PMC11005345
- DOI: 10.1128/mbio.00199-24
Human otic progenitor cell models of congenital hearing loss reveal potential pathophysiologic mechanisms of Zika virus and cytomegalovirus infections
Abstract
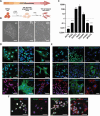
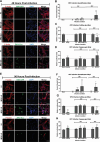
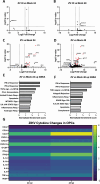
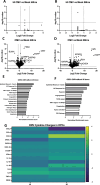
Congenital hearing loss is a common chronic condition affecting children in both developed and developing nations. Viruses correlated with congenital hearing loss include human cytomegalovirus (HCMV) and Zika virus (ZIKV), which causes congenital Zika syndrome. The mechanisms by which HCMV and ZIKV infections cause hearing loss are poorly understood. It is challenging to study human inner ear cells because they are encased in bone and also scarce as autopsy samples. Recent advances in culturing human stem cell-derived otic progenitor cells (OPCs) have allowed us herein to describe successful in vitro infection of OPCs with HCMV and ZIKV, and also to propose potential mechanisms by which each viral infection could affect hearing. We find that ZIKV infection rapidly and significantly induces the expression of type I interferon and interferon-stimulated genes, while OPC viability declines, at least in part, from apoptosis. In contrast, HCMV infection did not appear to upregulate interferons or cause a reduction in cell viability, and instead disrupted expression of key genes and pathways associated with inner ear development and function, including Cochlin, nerve growth factor receptor, SRY-box transcription factor 11, and transforming growth factor-beta signaling. These findings suggest that ZIKV and HCMV infections cause congenital hearing loss through distinct pathways, that is, by inducing progenitor cell death in the case of ZIKV infection, and by disruption of critical developmental pathways in the case of HCMV infection.
Importance: Congenital virus infections inflict substantial morbidity and devastating disease in neonates worldwide, and hearing loss is a common outcome. It has been difficult to study viral infections of the human hearing apparatus because it is embedded in the temporal bone of the skull. Recent technological advances permit the differentiation of otic progenitor cells (OPCs) from human-induced pluripotent stem cells. This paper is important for demonstrating that inner ear virus infections can be modeled in vitro using OPCs. We infected OPCs with two viruses associated with congenital hearing loss: human cytomegalovirus (HCMV), a DNA virus, or Zika virus (ZIKV), an RNA virus. An important result is that the gene expression and cytokine production profiles of HCMV/ZIKV-infected OPCs are markedly dissimilar, suggesting that mechanisms of hearing loss are also distinct. The specific molecular regulatory pathways identified in this work could suggest important targets for therapeutics.
Keywords: Zika virus; hearing loss; human cytomegalovirus; inner ear; model system; organoid; virus infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Zika Virus Infects Early- and Midgestation Human Maternal Decidual Tissues, Inducing Distinct Innate Tissue Responses in the Maternal-Fetal Interface.J Virol. 2017 Jan 31;91(4):e01905-16. doi: 10.1128/JVI.01905-16. Print 2017 Feb 15. J Virol. 2017. PMID: 27974560 Free PMC article.
-
Zika virus infection causes widespread damage to the inner ear.Hear Res. 2020 Sep 15;395:108000. doi: 10.1016/j.heares.2020.108000. Epub 2020 Jun 29. Hear Res. 2020. PMID: 32623238 Free PMC article.
-
Changes in ADAR RNA editing patterns in CMV and ZIKV congenital infections.BMC Genomics. 2023 Nov 15;24(1):685. doi: 10.1186/s12864-023-09778-4. BMC Genomics. 2023. PMID: 37968596 Free PMC article.
-
Congenital Cytomegalovirus and Zika Infections.Indian J Pediatr. 2020 Oct;87(10):840-845. doi: 10.1007/s12098-020-03260-9. Epub 2020 Apr 13. Indian J Pediatr. 2020. PMID: 32281058 Review.
-
Pluripotent Stem Cell-Based Models: A Peephole into Virus Infections during Early Pregnancy.Cells. 2020 Feb 26;9(3):542. doi: 10.3390/cells9030542. Cells. 2020. PMID: 32110999 Free PMC article. Review.
Cited by
-
Application of diceCT to Study the Development of the Zika Virus-Infected Mouse Brain.Viruses. 2024 Aug 20;16(8):1330. doi: 10.3390/v16081330. Viruses. 2024. PMID: 39205304 Free PMC article.
-
The Effects of Viral Infections on the Molecular and Signaling Pathways Involved in the Development of the PAOs.Viruses. 2024 Aug 22;16(8):1342. doi: 10.3390/v16081342. Viruses. 2024. PMID: 39205316 Free PMC article. Review.
-
Congenital Zika virus infection in laboratory animals: a comparative review highlights translational studies on the maternal-foetal interface.Mem Inst Oswaldo Cruz. 2025 Feb 28;120:e240125. doi: 10.1590/0074-02760240125. eCollection 2025. Mem Inst Oswaldo Cruz. 2025. PMID: 40052994 Free PMC article. Review.
References
-
- Lanzieri TM, Leung J, Caviness AC, Chung W, Flores M, Blum P, Bialek SR, Miller JA, Vinson SS, Turcich MR, Voigt RG, Demmler-Harrison G, for the Congenital Cytomegalovirus Longitudinal Study Group . 2017. Long-term outcomes of children with symptomatic congenital cytomegalovirus disease. J Perinatol 37:875–880. doi:10.1038/jp.2017.41 - DOI - PMC - PubMed
-
- Teissier N, Delezoide A-L, Mas A-E, Khung-Savatovsky S, Bessières B, Nardelli J, Vauloup-Fellous C, Picone O, Houhou N, Oury J-F, Van Den Abbeele T, Gressens P, Adle-Biassette H. 2011. Inner ear lesions in congenital cytomegalovirus infection of human fetuses. Acta Neuropathol 122:763–774. doi:10.1007/s00401-011-0895-y - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

