Turmeric-Derived Nanoparticles Functionalized Aerogel Regulates Multicellular Networks to Promote Diabetic Wound Healing
- PMID: 38441389
- PMCID: PMC11095230
- DOI: 10.1002/advs.202307630
Turmeric-Derived Nanoparticles Functionalized Aerogel Regulates Multicellular Networks to Promote Diabetic Wound Healing
Abstract
Regulation of excessive inflammation and impaired cell proliferation is crucial for healing diabetic wounds. Although plant-to-mammalian regulation offers effective approaches for chronic wound management, the development of a potent plant-based therapeutic presents challenges. This study aims to validate the efficacy of turmeric-derived nanoparticles (TDNPs) loaded with natural bioactive compounds. TDNPs can alleviate oxidative stress, promote fibroblast proliferation and migration, and reprogram macrophage polarization. Restoration of the fibroblast-macrophage communication network by TDNPs stimulates cellular regeneration, in turn enhancing diabetic wound healing. To address diabetic wound management, TDNPs are loaded in an ultralight-weight, high swelling ratio, breathable aerogel (AG) constructed with cellulose nanofibers and sodium alginate backbones to obtain TDNPs@AG (TAG). TAG features wound shape-customized accessibility, water-adaptable tissue adhesiveness, and capacity for sustained release of TDNPs, exhibiting outstanding performance in facilitating in vivo diabetic wound healing. This study highlights the potential of TDNPs in regenerative medicine and their applicability as a promising solution for wound healing in clinical settings.
Keywords: Nrf2/HO‐1 pathway; aerogels; cross‐kingdom regulations; diabetic wound healing; turmeric‐derived nanoparticles.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


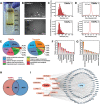
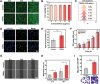





References
-
- Sun H., Saeedi P., Karuranga S., Pinkepank M., Ogurtsova K., Duncan B. B., Stein C., Basit A., Chan J. C. N., Mbanya J. C., Pavkov M. E., Ramachandaran A., Wild S. H., James S., Herman W. H., Zhang P., Bommer C., Kuo S., Boyko E. J., Magliano D. J., Diabetes Res. Clin. Pract. 2022, 183, 109119. - PMC - PubMed
-
- Jeffcoate W. J., Harding K. G., Lancet 2003, 361, 1545. - PubMed
-
- Hart T., Milner R., Cifu A., JAMA, J. Am. Med. Assoc. 2017, 318, 1387. - PubMed
-
- Falanga V., Lancet 2005, 366, 1736. - PubMed
MeSH terms
Grants and funding
- 81871735/National Natural Science Foundation of China
- 82172371/National Natural Science Foundation of China
- 82302639/National Natural Science Foundation of China
- 2023M731555/China Postdoctoral Science Foundation
- 2020J007/Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University
LinkOut - more resources
Full Text Sources
