Escherichia coli possessing the dihydroxyacetone phosphate shunt utilize 5'-deoxynucleosides for growth
- PMID: 38441472
- PMCID: PMC10986504
- DOI: 10.1128/spectrum.03086-23
Escherichia coli possessing the dihydroxyacetone phosphate shunt utilize 5'-deoxynucleosides for growth
Abstract
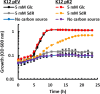
All organisms utilize S-adenosyl-l-methionine (SAM) as a key co-substrate for the methylation of biological molecules, the synthesis of polyamines, and radical SAM reactions. When these processes occur, 5'-deoxy-nucleosides are formed as byproducts such as S-adenosyl-l-homocysteine, 5'-methylthioadenosine (MTA), and 5'-deoxyadenosine (5dAdo). A prevalent pathway found in bacteria for the metabolism of MTA and 5dAdo is the dihydroxyacetone phosphate (DHAP) shunt, which converts these compounds into dihydroxyacetone phosphate and 2-methylthioacetaldehyde or acetaldehyde, respectively. Previous work in other organisms has shown that the DHAP shunt can enable methionine synthesis from MTA or serve as an MTA and 5dAdo detoxification pathway. Rather, the DHAP shunt in Escherichia coli ATCC 25922, when introduced into E. coli K-12, enables the use of 5dAdo and MTA as a carbon source for growth. When MTA is the substrate, the sulfur component is not significantly recycled back to methionine but rather accumulates as 2-methylthioethanol, which is slowly oxidized non-enzymatically under aerobic conditions. The DHAP shunt in ATCC 25922 is active under oxic and anoxic conditions. Growth using 5-deoxy-d-ribose was observed during aerobic respiration and anaerobic respiration with Trimethylamine N-oxide (TMAO), but not during fermentation or respiration with nitrate. This suggests the DHAP shunt may only be relevant for extraintestinal pathogenic E. coli lineages with the DHAP shunt that inhabit oxic or TMAO-rich extraintestinal environments. This reveals a heretofore overlooked role of the DHAP shunt in carbon and energy metabolism from ubiquitous SAM utilization byproducts and suggests a similar role may occur in other pathogenic and non-pathogenic bacteria with the DHAP shunt.
Importance: The acquisition and utilization of organic compounds that serve as growth substrates are essential for Escherichia coli to grow and multiply. Ubiquitous enzymatic reactions involving S-adenosyl-l-methionine as a co-substrate by all organisms result in the formation of the 5'-deoxy-nucleoside byproducts, 5'-methylthioadenosine and 5'-deoxyadenosine. All E. coli possess a conserved nucleosidase that cleaves these 5'-deoxy-nucleosides into 5-deoxy-pentose sugars for adenine salvage. The DHAP shunt pathway is found in some extraintestinal pathogenic E. coli, but its function in E. coli possessing it has remained unknown. This study reveals that the DHAP shunt enables the utilization of 5'-deoxy-nucleosides and 5-deoxy-pentose sugars as growth substrates in E. coli strains with the pathway during aerobic respiration and anaerobic respiration with TMAO, but not fermentative growth. This provides an insight into the diversity of sugar compounds accessible by E. coli with the DHAP shunt and suggests that the DHAP shunt is primarily relevant in oxic or TMAO-rich extraintestinal environments.
Keywords: carbon metabolism; extraintestinal pathogenic E. coli; nucleotide metabolism; sulfur.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Utilization of 5'-deoxy-nucleosides as Growth Substrates by Extraintestinal Pathogenic E. coli via the Dihydroxyacetone Phosphate Shunt.bioRxiv [Preprint]. 2023 Aug 10:2023.08.10.552779. doi: 10.1101/2023.08.10.552779. bioRxiv. 2023. PMID: 37609188 Free PMC article. Preprint.
-
A bifunctional salvage pathway for two distinct S-adenosylmethionine by-products that is widespread in bacteria, including pathogenic Escherichia coli.Mol Microbiol. 2020 May;113(5):923-937. doi: 10.1111/mmi.14459. Epub 2020 Feb 20. Mol Microbiol. 2020. PMID: 31950558 Free PMC article.
-
Two Distinct Aerobic Methionine Salvage Pathways Generate Volatile Methanethiol in Rhodopseudomonas palustris.mBio. 2018 Apr 10;9(2):e00407-18. doi: 10.1128/mBio.00407-18. mBio. 2018. PMID: 29636438 Free PMC article.
-
5-Deoxyadenosine Metabolism: More than "Waste Disposal".Microb Physiol. 2021;31(3):248-259. doi: 10.1159/000516105. Epub 2021 Jun 14. Microb Physiol. 2021. PMID: 34126623 Review.
-
Metabolic characteristics and importance of the universal methionine salvage pathway recycling methionine from 5'-methylthioadenosine.IUBMB Life. 2009 Dec;61(12):1132-42. doi: 10.1002/iub.278. IUBMB Life. 2009. PMID: 19946895 Review.
Cited by
-
Use of fecal microbiome to understand the impact of housing conditions on metabolic stress responses in farmed saltwater crocodiles (Crocodylus porosus).Front Vet Sci. 2025 Feb 13;12:1496946. doi: 10.3389/fvets.2025.1496946. eCollection 2025. Front Vet Sci. 2025. PMID: 40018705 Free PMC article.
-
Carboxy-Amidated AamAP1-Lys has Superior Conformational Flexibility and Accelerated Killing of Gram-Negative Bacteria.Biochemistry. 2025 Feb 18;64(4):841-859. doi: 10.1021/acs.biochem.4c00580. Epub 2025 Jan 28. Biochemistry. 2025. PMID: 39873636 Free PMC article.
References
-
- Owrangi B, Masters N, Kuballa A, O’Dea C, Vollmerhausen TL, Katouli M. 2018. Invasion and translocation of uropathogenic Escherichia coli isolated from urosepsis and patients with community-acquired urinary tract infection. Eur J Clin Microbiol Infect Dis 37:833–839. doi:10.1007/s10096-017-3176-4 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

