Antigen-Mimic Nanoparticles in Ultrasensitive on-Chip Integrated Anti-p53 Antibody Quantification
- PMID: 38441485
- PMCID: PMC10964233
- DOI: 10.1021/acssensors.3c02568
Antigen-Mimic Nanoparticles in Ultrasensitive on-Chip Integrated Anti-p53 Antibody Quantification
Abstract
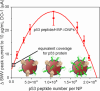
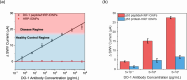
As a tumor-suppressing protein, p53 plays a crucial role in preventing cancer development. Its utility as an early cancer detection tool is significant, potentially enabling clinicians to forestall disease advancement and improve patient prognosis. In response to the pathological overexpression of this antigen in tumors, the prevalence of anti-p53 antibodies increases in serum, in a manner quantitatively indicative of cancer progression. This spike can be detected through techniques, such as Western blotting, immunohistochemistry, and immunoprecipitation. In this study, we present an electrochemical approach that supports ultrasensitive and highly selective anti-p53 autoantibody quantification without the use of an immuno-modified electrode. We specifically employ antigen-mimicking and antibody-capturing peptide-coated magnetic nanoparticles, along with an AC magnetic field-promoted sample mixing, prior to the presentation of Fab-captured targets to simple lectin-modified sensors. The subfemtomolar assays are highly selective and support quantification from serum-spiked samples within minutes.
Keywords: antigen-mimicking peptide; cancer detection; electrochemical enzyme-amplified assay; nanoparticle-assisted immunoisolation; p53.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Simplified approaches for the development of an ELISA to detect circulating autoantibodies to p53 in cancer patients.BMC Biotechnol. 2008 Feb 20;8:16. doi: 10.1186/1472-6750-8-16. BMC Biotechnol. 2008. PMID: 18284706 Free PMC article.
-
Evaluation of serum autoantibodies against tumor-associated antigens as biomarkers in lung cancer.Tumour Biol. 2017 Oct;39(10):1010428317711662. doi: 10.1177/1010428317711662. Tumour Biol. 2017. PMID: 29022480
-
Gold-loaded nanoporous iron oxide nanocubes: a novel dispersible capture agent for tumor-associated autoantibody analysis in serum.Nanoscale. 2017 Jun 29;9(25):8805-8814. doi: 10.1039/c7nr03006a. Nanoscale. 2017. PMID: 28627551
-
Mutant p53 as an Antigen in Cancer Immunotherapy.Int J Mol Sci. 2020 Jun 8;21(11):4087. doi: 10.3390/ijms21114087. Int J Mol Sci. 2020. PMID: 32521648 Free PMC article. Review.
-
Autoantibody profiling for cancer detection.Clin Lab Med. 2009 Mar;29(1):31-46. doi: 10.1016/j.cll.2009.01.002. Clin Lab Med. 2009. PMID: 19389549 Review.
References
-
- Wild C. P.; Weiderpass E., Stewart B. W.. World Cancer Report: Cancer Research for Cancer Prevention; IARC press Lyon, 2020. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

