Sex impacts treatment decisions in multiple sclerosis
- PMID: 38441611
- PMCID: PMC11136719
- DOI: 10.1007/s00415-024-12270-y
Sex impacts treatment decisions in multiple sclerosis
Abstract
Background: Individual disease-modifying treatment (DMT) decisions might differ between female and male people with MS (pwMS).
Objective: To identify sex-related differences in DMT strategies over the past decades in a real-world setting.
Methods: In this cohort study, data from the Austrian Multiple Sclerosis Treatment Registry (AMSTR), a nationwide prospectively collected registry mandatory for reimbursement, were retrospectively analyzed. Of 4840 pwMS, those with relapsing-remitting MS, aged at least 18 years, who started DMT and had at least two clinical visits, were identified. At baseline, demographics, Expanded Disability Status Scale (EDSS) score, annualized relapse rate (ARR) in the prior 12 months and MRI lesion load were assessed. At follow-up, ARR, EDSS scores, and DMT were determined.
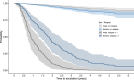
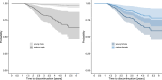
Results: A total of 4224 pwMS were included into the study and had a median of 10 (IQR 5-18) clinical visits over an observation period of 3.5 (IQR 1.5-6.1) years. Multivariable Cox regression analysis revealed that the probability of DMT escalation due to relapse activity was lower in female than male pwMS (HR 4.1 vs. 8.3 per ARR). Probability of discontinuing moderate-effective DMT was higher in female pwMS when they were younger (HR 1.03 per year), and lower in male pwMS at higher age (HR 0.92). Similarly, female pwMS were more likely to stop highly effective DMT than male pwMS (HR 1.7). Among others, the most frequent reason for DMT discontinuation was family planning in female pwMS. All sex-related effects were independent of disease activity, such as MRI lesion load, baseline ARR or EDSS.
Conclusions: Real-world treatment decisions are influenced by sex-related aspects. Awareness of these associations should prevent unwarranted differences in MS care.
Keywords: Gender; Multiple sclerosis; Registry; Sex; Treatment.
© 2024. The Author(s).
Conflict of interest statement
HH has participated in meetings sponsored by, received speaker honoraria or travel funding from Bayer, Biogen, Bristol Myers Squibb, Janssen, Merck, Novartis, Sanofi-Genzyme, Siemens, Teva, and received honoraria for acting as consultant for Biogen, Bristol Myers Squibb, Novartis, Roche, Sanofi-Genzyme and Teva. He is associate editor of Frontiers in Neurology. KB has participated in meetings sponsored by, received speaking honoraria or travel funding from Roche, Biogen, Sanofi and Teva. FD has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Alexion, Almirall, Biogen, Celgene-BMS, Genzyme-Sanofi, Horizon, Merck, Novartis Pharma, Roche, and Teva. His institution has received research grants from Biogen and Genzyme Sanofi. He is section editor of the MSARD Journal (Multiple Sclerosis and Related Disorders) and review editor of Frontiers Neurology. TB has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for multiple sclerosis: Almirall, Biogen, Bionorica, BMS/Celgene, Eisai, Horizon, Jazz Pharmaceuticals, Janssen-Cilag, MedDay, Merck, Novartis, Roche, Sanofi Aventis/Genzyme, Sandoz, TG Therapeutics, TEVA and UCB. His institution has received financial support in the last 2 years by unrestricted research grants (Biogen, BMS/Celgene, Novartis, Sanofi Aventis/Genzyme, Roche, TEVA) and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, BMS/Celgene, Merck, Novartis, Roche, Sanofi Aventis/Genzyme, TEVA. CE received funding for travel and speaker honoraria from Biogen, Bayer, Celgene, Merck, Novartis, Roche, Shire, Genzyme, and Teva Pharmaceutical Industries Ltd./sanofi-aventis; re- search support from Merck Serono, Biogen, and Teva Pharmaceutical Industries Ltd./sanofi-aventis; serving on scientific advisory boards for Bayer, Biogen, Celgene, Merck, Novartis, Roche and Teva Pharmaceutical Industries Ltd./sanofi-aventis. MG received support and honoraria for research, consultation, lectures and education from Alexion, Almirall, Bayer, Biogen, Bristol-Myers-Squibb, Celgene, Genzyme, Horizon, Janssen-Cilag, MedDay, Merck, Novartis, Roche, Sanofi Aventis and TEVA ratiopharm. JK received consulting and/or research funding and/ or educational support from Almirall, Bayer, Biogen, Celgene/ Bristol Myers Squibb, MedDay, Medtronic, Merck, Novartis, Roche, Sanofi- Aventis, Shire, and TEVA ratiopharm. JW has nothing to disclose. FDP has participated in meetings sponsored by, received honoraria (lectures, advisory boards, consultations) or travel funding from Bayer, Biogen, Celgene, Merck, Novartis, Sanofi-Genzyme, Roche and Teva.
Figures

Similar articles
-
Teriflunomide for multiple sclerosis.Cochrane Database Syst Rev. 2016 Mar 22;3(3):CD009882. doi: 10.1002/14651858.CD009882.pub3. Cochrane Database Syst Rev. 2016. PMID: 27003123 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2024 Jan 4;1(1):CD011381. doi: 10.1002/14651858.CD011381.pub3. Cochrane Database Syst Rev. 2024. PMID: 38174776 Free PMC article.
-
Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2013 Jun 6;2013(6):CD008933. doi: 10.1002/14651858.CD008933.pub2. Cochrane Database Syst Rev. 2013. PMID: 23744561 Free PMC article.
-
Rituximab for relapsing-remitting multiple sclerosis.Cochrane Database Syst Rev. 2013 Dec 6;2013(12):CD009130. doi: 10.1002/14651858.CD009130.pub3. Cochrane Database Syst Rev. 2013. PMID: 24310855 Free PMC article.
References
-
- Kalincik T, Vivek V, Jokubaitis V, Lechner-Scott J, Trojano M, Izquierdo G, Lugaresi A, Grand'maison F, Hupperts R, Oreja-Guevara C, Bergamaschi R, Iuliano G, Alroughani R, Van Pesch V, Amato MP, Slee M, Verheul F, Fernandez-Bolanos R, Fiol M, Spitaleri DL, Cristiano E, Gray O, Cabrera-Gomez JA, Shaygannejad V, Herbert J, Vucic S, Needham M, Petkovska-Boskova T, Sirbu CA, Duquette P, Girard M, Grammond P, Boz C, Giuliani G, Rio ME, Barnett M, Flechter S, Moore F, Singhal B, Bacile EA, Saladino ML, Shaw C, Skromne E, Poehlau D, Vella N, Spelman T, Liew D, Kilpatrick TJ, Butzkueven H, Group MS. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain. 2013;136:3609–3617. doi: 10.1093/brain/awt281. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

