Near-Infrared Perylenecarboximide Fluorophores for Live-Cell Super-Resolution Imaging
- PMID: 38441879
- PMCID: PMC10958508
- DOI: 10.1021/jacs.3c13368
Near-Infrared Perylenecarboximide Fluorophores for Live-Cell Super-Resolution Imaging
Abstract
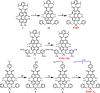
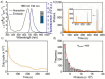
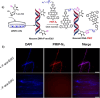
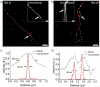
Organic near-infrared (NIR) photoblinking fluorophores are highly desirable for live-cell super-resolution imaging based on single-molecule localization microscopy (SMLM). Herein we introduce a novel small chromophore, PMIP, through the fusion of perylenecarboximide with 2,2-dimetheylpyrimidine. PMIP exhibits an emission maximum at 732 nm with a high fluorescence quantum yield of 60% in the wavelength range of 700-1000 nm and excellent photoblinking without any additives. With resorcinol-functionalized PMIP (PMIP-OH), NIR SMLM imaging of lysosomes is demonstrated for the first time in living mammalian cells under physiological conditions. Moreover, metabolically labeled nascent DNA is site-specifically detected using azido-functionalized PMIP (PMIP-N3) via click chemistry, thereby enabling the super-resolution imaging of nascent DNA in phosphate-buffered saline with a 9-fold improvement in spatial resolution. These results indicate the potential of PMIP-based NIR blinking fluorophores for biological applications of SMLM.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Chen Y.; Wang S.; Zhang F. Near-infrared luminescence high-contrast in vivo biomedical imaging. Nat. Rev. Bioeng. 2023, 1 (1), 60–78. 10.1038/s44222-022-00002-8. - DOI
-
- Casutt M.; Ruscello M.; Strobel N.; Koser S.; Bunz U. H. F.; Jänsch D.; Freudenberg J.; Hernandez-Sosa G.; Müllen K. Diketopyrrolopyrrole-Polymer Meets Thiol-Ene Click Chemistry: A Cross-Linked Acceptor for Thermally Stable Near-Infrared Photodetectors. Chem. Mater. 2019, 31 (18), 7657–7665. 10.1021/acs.chemmater.9b02530. - DOI
-
- Hong G.; Antaris A. L.; Dai H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1 (1), 0010.10.1038/s41551-016-0010. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

