Nurse-Led Strategy to Improve Blood Pressure and Cholesterol Level Among People With HIV: A Randomized Clinical Trial
- PMID: 38441897
- PMCID: PMC10915684
- DOI: 10.1001/jamanetworkopen.2023.56445
Nurse-Led Strategy to Improve Blood Pressure and Cholesterol Level Among People With HIV: A Randomized Clinical Trial
Abstract
Importance: Despite higher atherosclerotic cardiovascular disease (ASCVD) risk, people with HIV (PWH) experience unique barriers to ASCVD prevention, such as changing models of HIV primary care.
Objective: To test whether a multicomponent nurse-led strategy would improve systolic blood pressure (SBP) and non-high-density lipoprotein (HDL) cholesterol level in a diverse population of PWH receiving antiretroviral therapy (ART).
Design, setting, and participants: This randomized clinical trial enrolled PWH at 3 academic HIV clinics in the US from September 2019 to January 2022 and conducted follow-up for 12 months until January 2023. Included patients were 18 years or older and had a confirmed HIV diagnosis, an HIV-1 viral load less than 200 copies/mL, and both hypertension and hypercholesterolemia. Participants were stratified by trial site and randomized 1:1 to either the multicomponent EXTRA-CVD (A Nurse-Led Intervention to Extend the HIV Treatment Cascade for Cardiovascular Disease Prevention) intervention group or the control group. Primary analyses were conducted according to the intention-to-treat principle.
Intervention: The EXTRA-CVD group received home BP monitoring guidance and BP and cholesterol management from a dedicated prevention nurse at 4 in-person visits (baseline and 4, 8, and 12 months) and frequent telephone check-ins up to every 2 weeks as needed. The control group received general prevention education sessions from the prevention nurse at each of the 4 in-person visits.
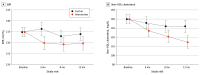
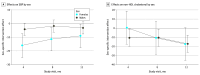
Main outcomes and measures: Study-measured SBP was the primary outcome, and non-HDL cholesterol level was the secondary outcome. Measurements were taken over 12 months and assessed by linear mixed models. Prespecified moderators tested were sex at birth, baseline ASCVD risk, and trial site.
Results: A total of 297 PWH were randomized to the EXTRA-CVD arm (n = 149) or control arm (n = 148). Participants had a median (IQR) age of 59.0 (53.0-65.0) years and included 234 males (78.8%). Baseline mean (SD) SBP was 135.0 (18.8) mm Hg and non-HDL cholesterol level was 139.9 (44.6) mg/dL. At 12 months, participants in the EXTRA-CVD arm had a clinically significant 4.2-mm Hg (95% CI, 0.3-8.2 mm Hg; P = .04) lower SBP and 16.9-mg/dL (95% CI, 8.6-25.2 mg/dL; P < .001) lower non-HDL cholesterol level compared with participants in the control arm. There was a clinically meaningful but not statistically significant difference in SBP effect in females compared with males (11.8-mm Hg greater difference at 4 months, 9.6 mm Hg at 8 months, and 5.9 mm Hg at 12 months; overall joint test P = .06).
Conclusions and relevance: Results of this trial indicate that the EXTRA-CVD strategy effectively reduced BP and cholesterol level over 12 months and should inform future implementation of multifaceted ASCVD prevention programs for PWH.
Trial registration: ClinicalTrials.gov Identifier: NCT03643705.
Conflict of interest statement
Figures
References
-
- Althoff KN, Gebo KA, Moore RD, et al. ; North American AIDS Cohort Collaboration on Research and Design . Contributions of traditional and HIV-related risk factors on non-AIDS-defining cancer, myocardial infarction, and end-stage liver and renal diseases in adults with HIV in the USA and Canada: a collaboration of cohort studies. Lancet HIV. 2019;6(2):e93-e104. doi:10.1016/S2352-3018(18)30295-9 - DOI - PMC - PubMed

