Heart failure pharmacotherapy and cancer: pathways and pre-clinical/clinical evidence
- PMID: 38441940
- PMCID: PMC11023004
- DOI: 10.1093/eurheartj/ehae105
Heart failure pharmacotherapy and cancer: pathways and pre-clinical/clinical evidence
Abstract
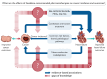
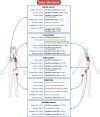
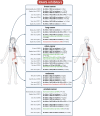
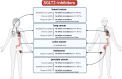
Heart failure (HF) patients have a significantly higher risk of new-onset cancer and cancer-associated mortality, compared to subjects free of HF. While both the prevention and treatment of new-onset HF in patients with cancer have been investigated extensively, less is known about the prevention and treatment of new-onset cancer in patients with HF, and whether and how guideline-directed medical therapy (GDMT) for HF should be modified when cancer is diagnosed in HF patients. The purpose of this review is to elaborate and discuss the effects of pillar HF pharmacotherapies, as well as digoxin and diuretics on cancer, and to identify areas for further research and novel therapeutic strategies. To this end, in this review, (i) proposed effects and mechanisms of action of guideline-directed HF drugs on cancer derived from pre-clinical data will be described, (ii) the evidence from both observational studies and randomized controlled trials on the effects of guideline-directed medical therapy on cancer incidence and cancer-related outcomes, as synthetized by meta-analyses will be reviewed, and (iii) considerations for future pre-clinical and clinical investigations will be provided.
Keywords: Angiotensin receptor blocker; Angiotensin receptor-neprilysin inhibitor; Angiotensin-converting enzyme inhibitor; Beta-blocker; Cancer; Cardio-oncology; Heart failure; Mineralocorticoid Receptor antagonist; Sodium-glucose cotransporter 2 inhibitor.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures



References
-
- Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol 2022;79:e263–421. doi:10.1016/j.jacc.2021.12.012 - DOI - PubMed
-
- Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS): developed by the T. Eur Heart J 2022;43:4229–361. 10.1093/eurheartj/ehac244 - DOI - PubMed
-
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1789–858. 10.1016/S0140-6736(18)32279-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

