CD93 maintains endothelial barrier function and limits metastatic dissemination
- PMID: 38441970
- PMCID: PMC11128212
- DOI: 10.1172/jci.insight.169830
CD93 maintains endothelial barrier function and limits metastatic dissemination
Abstract
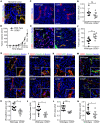
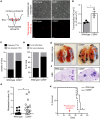
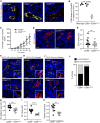
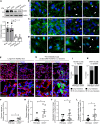
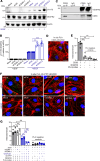
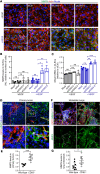
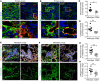
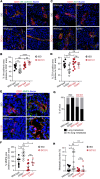
Compromised vascular integrity facilitates extravasation of cancer cells and promotes metastatic dissemination. CD93 has emerged as a target for antiangiogenic therapy, but its importance for vascular integrity in metastatic cancers has not been evaluated. Here, we demonstrate that CD93 participates in maintaining the endothelial barrier and reducing metastatic dissemination. Primary melanoma growth was hampered in CD93-/- mice, but metastatic dissemination was increased and associated with disruption of adherens and tight junctions in tumor endothelial cells and elevated expression of matrix metalloprotease 9 at the metastatic site. CD93 directly interacted with vascular endothelial growth factor receptor 2 (VEGFR2) and its absence led to VEGF-induced hyperphosphorylation of VEGFR2 in endothelial cells. Antagonistic anti-VEGFR2 antibody therapy rescued endothelial barrier function and reduced the metastatic burden in CD93-/- mice to wild-type levels. These findings reveal a key role of CD93 in maintaining vascular integrity, which has implications for pathological angiogenesis and endothelial barrier function in metastatic cancer.
Keywords: Endothelial cells; Extracellular matrix; Melanoma; Oncology; Vascular biology.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

