Heme oxygenase-1 is an equid alphaherpesvirus 8 replication restriction host protein and suppresses viral replication via the PKCβ/ERK1/ERK2 and NO/cGMP/PKG pathway
- PMID: 38441979
- PMCID: PMC10986571
- DOI: 10.1128/spectrum.03220-23
Heme oxygenase-1 is an equid alphaherpesvirus 8 replication restriction host protein and suppresses viral replication via the PKCβ/ERK1/ERK2 and NO/cGMP/PKG pathway
Abstract
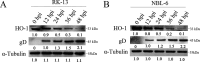
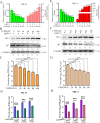
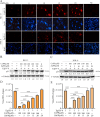
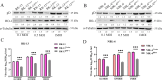
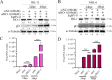
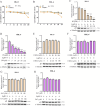
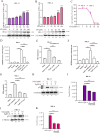
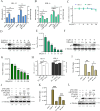
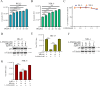
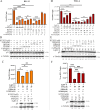
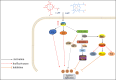
Equid alphaherpesvirus 8 (EqHV-8) is one of the most economically important viruses that is known to cause severe respiratory disease, abortion, and neurological syndromes in equines. However, no effective vaccines or therapeutic agents are available to control EqHV-8 infection. Heme oxygenase-1 (HO-1) is an antioxidant defense enzyme that displays significant cytoprotective effects against different viral infections. However, the literature on the function of HO-1 during EqHV-8 infection is little. We explored the effects of HO-1 on EqHV-8 infection and revealed its potential mechanisms. Our results demonstrated that HO-1 induced by cobalt-protoporphyrin (CoPP) or HO-1 overexpression inhibited EqHV-8 replication in susceptible cells. In contrast, HO-1 inhibitor (zinc protoporphyria) or siRNA targeting HO-1 reversed the anti-EqHV-8 activity. Furthermore, biliverdin, a metabolic product of HO-1, mediated the anti-EqHV-8 effect of HO-1 via both the protein kinase C (PKC)β/extracellular signal-regulated kinase (ERK)1/ERK2 and nitric oxide (NO)-dependent cyclic guanosine monophosphate (cGMP)-protein kinase G (PKG) signaling pathways. In addition, CoPP protected the mice by reducing the EqHV-8 infection in the lungs. Altogether, these results indicated that HO-1 can be developed as a promising therapeutic strategy to control EqHV-8 infection.IMPORTANCEEqHV-8 infections have threatened continuously donkey and horse industry worldwide, which induces huge economic losses every year. However, no effective vaccination strategies or drug against EqHV-8 infection until now. Our present study found that one host protien HO-1 restrict EqHV-8 replication in vitro and in vivo. Furthermore, we demonstrate that HO-1 and its metabolite biliverdin suppress EqHV-8 relication via the PKCβ/ERK1/ERK2 and NO/cGMP/PKG pathways. Hence, we believe that HO-1 can be developed as a promising therapeutic strategy to control EqHV-8 infection.
Keywords: EqHV-8; HO-1; anti-viral effect; biliverdin; mouse model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Cobalt Protoporphyrin Blocks EqHV-8 Infection via IFN-α/β Production.Animals (Basel). 2023 Aug 22;13(17):2690. doi: 10.3390/ani13172690. Animals (Basel). 2023. PMID: 37684954 Free PMC article.
-
Heme Oxygenase-1 and Its Metabolites Carbon Monoxide and Biliverdin, but Not Iron, Exert Antiviral Activity against Porcine Circovirus Type 3.Microbiol Spectr. 2023 Jun 15;11(3):e0506022. doi: 10.1128/spectrum.05060-22. Epub 2023 May 4. Microbiol Spectr. 2023. PMID: 37140466 Free PMC article.
-
Heme Oxygenase-1 inhibits spring viremia of carp virus replication through carbon monoxide mediated cyclic GMP/Protein kinase G signaling pathway.Fish Shellfish Immunol. 2018 Aug;79:65-72. doi: 10.1016/j.fsi.2018.05.014. Epub 2018 May 9. Fish Shellfish Immunol. 2018. PMID: 29753142
-
[New paradigm of host defense against intracellular pathogens by nitric oxide].Nihon Hansenbyo Gakkai Zasshi. 2009 Feb;78(1):41-7. doi: 10.5025/hansen.78.41. Nihon Hansenbyo Gakkai Zasshi. 2009. PMID: 19227148 Review. Japanese.
-
Heme oxygenase system and hypertension: a comprehensive insight.Curr Pharm Des. 2014;20(9):1354-69. doi: 10.2174/13816128113199990558. Curr Pharm Des. 2014. PMID: 23978093 Review.
Cited by
-
An Overview of Infectious and Non-Infectious Causes of Pregnancy Losses in Equine.Animals (Basel). 2024 Jul 2;14(13):1961. doi: 10.3390/ani14131961. Animals (Basel). 2024. PMID: 38998073 Free PMC article. Review.
-
Blebbistatin as a novel antiviral agent targeting equid herpesvirus type 8.Front Vet Sci. 2024 Jun 5;11:1390304. doi: 10.3389/fvets.2024.1390304. eCollection 2024. Front Vet Sci. 2024. PMID: 38898998 Free PMC article.
-
Advances in Donkey Disease Surveillance and Microbiome Characterization in China.Microorganisms. 2025 Mar 26;13(4):749. doi: 10.3390/microorganisms13040749. Microorganisms. 2025. PMID: 40284586 Free PMC article. Review.
-
Hyperoside inhibits PRRSV proliferation via the TLR4/NF-κB and p62-Nrf2-Keap1 signaling pathways, mediating inflammation and autophagy.Microbiol Spectr. 2025 Aug 5;13(8):e0310724. doi: 10.1128/spectrum.03107-24. Epub 2025 Jun 12. Microbiol Spectr. 2025. PMID: 40503831 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- 32002248/MOST | National Natural Science Foundation of China (NSFC)
- ZR2020QC016/| Natural Science Foundation of Shandong Province ()
- ZR2020QC017/| Natural Science Foundation of Shandong Province ()
- 2022KJ287/The Project of Shandong Province Higher Educational Science and Technology Program for Youth
LinkOut - more resources
Full Text Sources
Miscellaneous

