Antagonistic effects of the cytotoxic molecules granzyme B and TRAIL in the immunopathogenesis of sclerosing cholangitis
- PMID: 38441998
- PMCID: PMC11407778
- DOI: 10.1097/HEP.0000000000000830
Antagonistic effects of the cytotoxic molecules granzyme B and TRAIL in the immunopathogenesis of sclerosing cholangitis
Abstract
Background and aims: Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease characterized by biliary inflammation and fibrosis. We showed an elevated interferon γ response in patients with primary sclerosing cholangitis and in multidrug resistance protein 2-deficient ( Mdr2-/- ) mice developing sclerosing cholangitis. Interferon γ induced expression of the cytotoxic molecules granzyme B (GzmB) and TRAIL in hepatic lymphocytes and mediated liver fibrosis in sclerosing cholangitis.
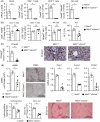
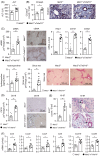
Approach and results: In patient samples and Mdr2-/- mice, we identified lymphocyte clusters with a cytotoxic gene expression profile using single-cell RNA-seq and cellular indexing of transcriptomes and epitopes by sequencing analyses combined with multi-parameter flow cytometry. CD8 + T cells and NK cells showed increased expression of GzmB and TRAIL in sclerosing cholangitis. Depletion of CD8 + T cells ameliorated disease severity in Mdr2-/- mice. By using Mdr2-/- × Gzmb-/- and Mdr2-/- × Tnfsf10-/- mice, we investigated the significance of GzmB and TRAIL for disease progression in sclerosing cholangitis. Interestingly, the lack of GzmB resulted in reduced cholangiocyte apoptosis, liver injury, and fibrosis. In contrast, sclerosing cholangitis was aggravated in the absence of TRAIL. This correlated with elevated GzmB and interferon γ expression by CD8 + T cells and NK cells enhanced T-cell survival, and increased apoptosis and expansion of cholangiocytes.
Conclusions: GzmB induces apoptosis and fibrosis in sclerosing cholangitis, whereas TRAIL regulates inflammatory and cytotoxic immune responses, subsequently leading to reduced liver injury and fibrosis.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Christoph Schramm consults for Agomab and Chemomab. He advises Pliant. He received grants from Falk and Roche. The remaining authors have no conflicts to report.
Figures




Comment in
-
Finding the TRAIL to escape granzyme B-mediated liver injury in PSC.Hepatology. 2024 Oct 1;80(4):770-772. doi: 10.1097/HEP.0000000000000859. Epub 2024 Mar 19. Hepatology. 2024. PMID: 38502805 Free PMC article. No abstract available.
References
-
- Karlsen TH, Boberg KM, Olsson M, Sun JY, Senitzer D, Bergquist A, et al. . Particular genetic variants of ligands for natural killer cell receptors may contribute to the HLA associated risk of primary sclerosing cholangitis. J Hepatol. 2007;46:899–906. - PubMed
-
- Kunzmann LK, Schoknecht T, Poch T, Henze L, Stein S, Kriz M, et al. . Monocytes as potential mediators of pathogen-induced T-helper 17 differentiation in patients with primary sclerosing cholangitis (PSC). Hepatology. 2020;72:1310–1326. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

