Recent Contributions of Proteomics to Our Understanding of Reversible Nε-Lysine Acylation in Bacteria
- PMID: 38442041
- PMCID: PMC11296938
- DOI: 10.1021/acs.jproteome.3c00912
Recent Contributions of Proteomics to Our Understanding of Reversible Nε-Lysine Acylation in Bacteria
Abstract
Post-translational modifications (PTMs) have been extensively studied in both eukaryotes and prokaryotes. Lysine acetylation, originally thought to be a rare occurrence in bacteria, is now recognized as a prevalent and important PTM in more than 50 species. This expansion in interest in bacterial PTMs became possible with the advancement of mass spectrometry technology and improved reagents such as acyl-modification specific antibodies. In this Review, we discuss how mass spectrometry-based proteomic studies of lysine acetylation and other acyl modifications have contributed to our understanding of bacterial physiology, focusing on recently published studies from 2018 to 2023. We begin with a discussion of approaches used to study bacterial PTMs. Next, we discuss newly characterized acylomes, including acetylomes, succinylomes, and malonylomes, in different bacterial species. In addition, we examine proteomic contributions to our understanding of bacterial virulence and biofilm formation. Finally, we discuss the contributions of mass spectrometry to our understanding of the mechanisms of acetylation, both enzymatic and nonenzymatic. We end with a discussion of the current state of the field and possible future research avenues to explore.
Keywords: KAT; KDAC; PTM; acetyl; acetylation; acetylome; post-translational modification; succinylation; succinylome.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
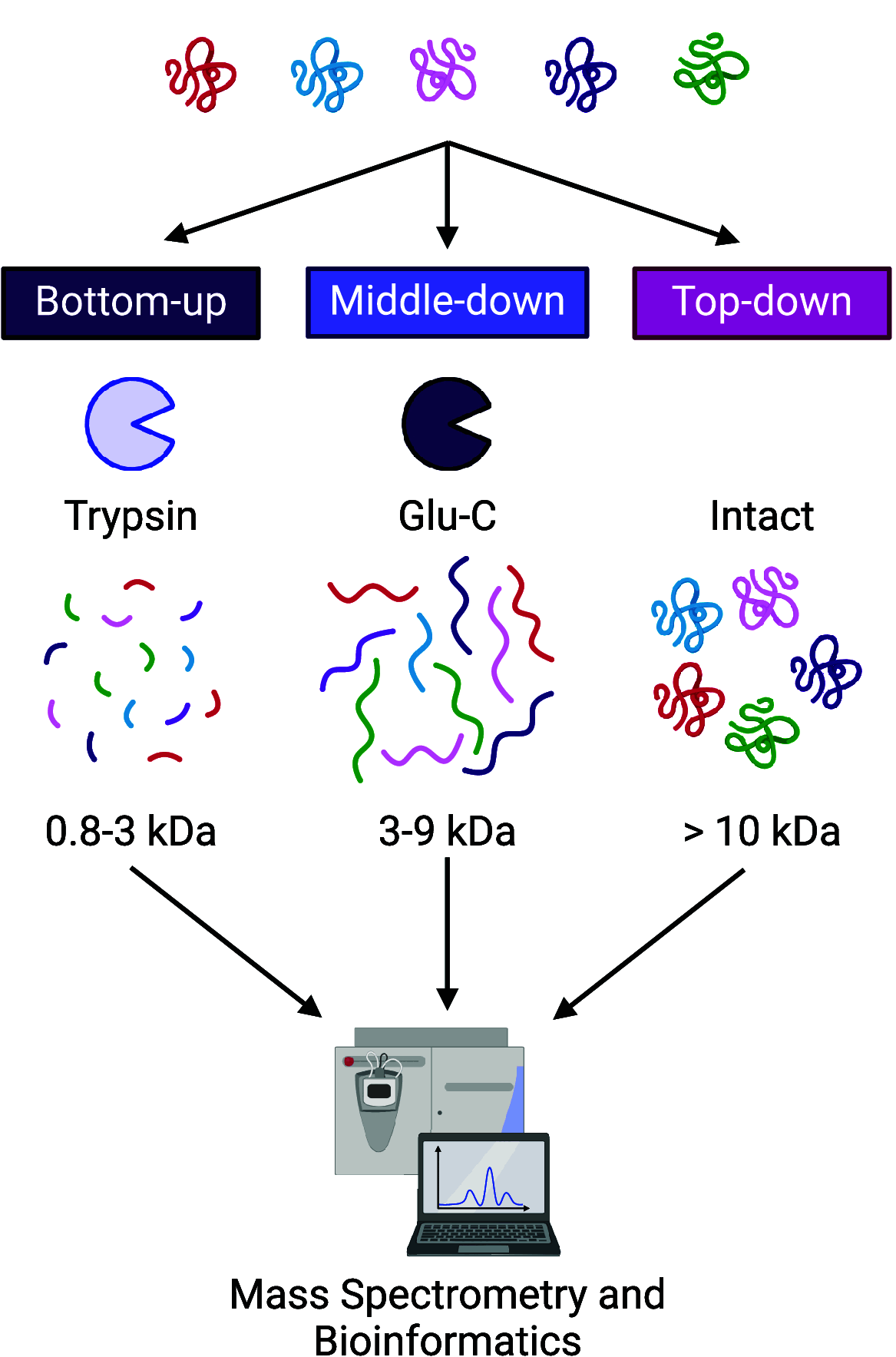


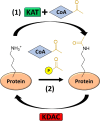
Similar articles
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Lysine acetylation in cyanobacteria: emerging mechanisms and functions.Biochem Soc Trans. 2025 Feb 12;53(1):BST20241037. doi: 10.1042/BST20241037. Biochem Soc Trans. 2025. PMID: 39936403 Free PMC article. Review.
-
Gender differences in the context of interventions for improving health literacy in migrants: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2024 Dec 12;12(12):CD013302. doi: 10.1002/14651858.CD013302.pub2. Cochrane Database Syst Rev. 2024. PMID: 39665382
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
Cited by
-
Lysine Phoshoglycerylation Is Widespread in Bacteria and Overlaps with Acylation.Microorganisms. 2024 Jul 30;12(8):1556. doi: 10.3390/microorganisms12081556. Microorganisms. 2024. PMID: 39203397 Free PMC article.
-
Innovative Formulation Strategies for Biosimilars: Trends Focused on Buffer-Free Systems, Safety, Regulatory Alignment, and Intellectual Property Challenges.Pharmaceuticals (Basel). 2025 Jun 17;18(6):908. doi: 10.3390/ph18060908. Pharmaceuticals (Basel). 2025. PMID: 40573303 Free PMC article. Review.
References
-
- Levene P. A.; Alsberg C. L. The cleavage products of vitellin. J. Biol. Chem. 1906, 2 (1), 127–133. 10.1016/S0021-9258(17)46054-6. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

