Distinctive antibody responses to Mycobacterium tuberculosis in pulmonary and brain infection
- PMID: 38442687
- PMCID: PMC11370789
- DOI: 10.1093/brain/awae066
Distinctive antibody responses to Mycobacterium tuberculosis in pulmonary and brain infection
Abstract
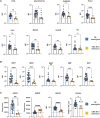
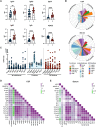
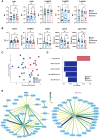
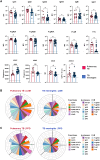
Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), remains a global health burden. While M. tuberculosis is primarily a respiratory pathogen, it can spread to other organs, including the brain and meninges, causing TB meningitis (TBM). However, little is known about the immunological mechanisms that lead to differential disease across organs. Attention has focused on differences in T cell responses in the control of M. tuberculosis in the lungs, but emerging data point to a role for antibodies, as both biomarkers of disease control and as antimicrobial molecules. Given an increasing appreciation for compartmentalized antibody responses across the blood-brain barrier, here we characterized the antibody profiles across the blood and brain compartments in TBM and determined whether M. tuberculosis-specific humoral immune responses differed between M. tuberculosis infection of the lung (pulmonary TB) and TBM. Using a high throughput systems serology approach, we deeply profiled the antibody responses against 10 different M. tuberculosis antigens, including lipoarabinomannan (LAM) and purified protein derivative (PPD), in HIV-negative adults with pulmonary TB (n = 10) versus TBM (n = 60). Antibody studies included analysis of immunoglobulin isotypes (IgG, IgM, IgA) and subclass levels (IgG1-4) and the capacity of M. tuberculosis-specific antibodies to bind to Fc receptors or C1q and to activate innate immune effector functions (complement and natural killer cell activation; monocyte or neutrophil phagocytosis). Machine learning methods were applied to characterize serum and CSF responses in TBM, identify prognostic factors associated with disease severity, and define the key antibody features that distinguish TBM from pulmonary TB. In individuals with TBM, we identified CSF-specific antibody profiles that marked a unique and compartmentalized humoral response against M. tuberculosis, characterized by an enrichment of M. tuberculosis-specific antibodies able to robustly activate complement and drive phagocytosis by monocytes and neutrophils, all of which were associated with milder TBM severity at presentation. Moreover, individuals with TBM exhibited M. tuberculosis-specific antibodies in the serum with an increased capacity to activate phagocytosis by monocytes, compared with individuals with pulmonary TB, despite having lower IgG titres and Fcγ receptor-binding capacity. Collectively, these data point to functionally divergent humoral responses depending on the site of infection (i.e. lungs versus brain) and demonstrate a highly compartmentalized M. tuberculosis-specific antibody response within the CSF in TBM. Moreover, our results suggest that phagocytosis- and complement-mediating antibodies may promote attenuated neuropathology and milder TBM disease.
Keywords: Fc receptors; TB meningitis; antibody-mediated complement deposition; antibody-mediated phagocytosis; neuroinflammation.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
G.A. is an employee of Moderna Therapeutics and holds equity in Leyden Labs and SeromYx Systems. The other authors report no competing interests.
Figures

References
-
- World Health Organization . Global Tuberculosis Report 2020. World Health Organization; 2020.
-
- Bartzatt R. Tuberculosis infections of the central nervous system. Cent. Nerv. Syst. Agents Med. Chem. 2011;11:321–327. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

