Chromosomal Microarray Analysis in Fetuses With Ultrasonographic Soft Markers: A Meta-Analysis of the Current Evidence
- PMID: 38442716
- PMCID: PMC10911939
- DOI: 10.3346/jkms.2024.39.e70
Chromosomal Microarray Analysis in Fetuses With Ultrasonographic Soft Markers: A Meta-Analysis of the Current Evidence
Abstract
Background: Ultrasonographic soft markers are normal variants, rather than fetal abnormalities, and guidelines recommend a detailed survey of fetal anatomy to determine the necessity of antenatal karyotyping. Anecdotal reports have described cases with ultrasonographic soft markers in which chromosomal microarray analysis (CMA) revealed pathogenic copy number variants (CNVs) despite normal results on conventional karyotyping, but CMA for ultrasonographic soft markers remains a matter of debate. In this systematic review, we evaluated the clinical significance of CMA for pregnancies with isolated ultrasonographic soft markers and a normal fetal karyotype.
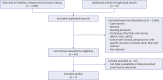
Methods: An electronic search was conducted by an experienced librarian through the MEDLINE, Embase, and Cochrane CENTRAL databases. We reviewed 3,338 articles (3,325 identified by database searching and 13 by a hand search) about isolated ultrasonographic soft markers, and seven ultrasonographic markers (choroid plexus cysts, echogenic bowel, echogenic intracardiac focus, hypoplastic nasal bone, short femur [SF], single umbilical artery, and urinary tract dilatation) were included for this study.
Results: Seven eligible articles were included in the final review. Pathogenic or likely pathogenic CNVs were found in fetuses with isolated ultrasonographic soft markers and a normal karyotype. The overall prevalence of pathogenic or likely pathogenic CNVs was 2.0% (41 of 2,048). The diagnostic yield of CMA was highest in fetuses with isolated SF (9 of 225, 3.9%).
Conclusion: CMA could aid in risk assessment and pregnancy counseling in pregnancies where the fetus has isolated ultrasonographic soft markers along with a normal karyotype.
Keywords: Chromosomal Microarray Analysis; Copy Number Variants; Fetal Chromosomal Abnormalities; Fetal Ultrasonography; Pregnancy; Ultrasonographic Soft Marker.
© 2024 The Korean Academy of Medical Sciences.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures
References
-
- Nyberg DA, Souter VL. Sonographic markers of fetal trisomies: second trimester. J Ultrasound Med. 2001;20(6):655–674. - PubMed
-
- Society for Maternal-Fetal Medicine (SMFM). Electronic address: pubs@smfm.org. Prabhu M, Kuller JA, Biggio JR. Society for Maternal-Fetal Medicine consult series #57: evaluation and management of isolated soft ultrasound markers for aneuploidy in the second trimester: (replaces consults #10, single umbilical artery, October 2010; #16, isolated echogenic bowel diagnosed on second-trimester ultrasound, August 2011; #17, evaluation and management of isolated renal pelviectasis on second-trimester ultrasound, December 2011; #25, isolated fetal choroid plexus cysts, April 2013; #27, isolated echogenic intracardiac focus, August 2013) Am J Obstet Gynecol. 2021;225(4):B2–15. - PubMed
-
- Cunningham FG, Leveno KJ, Dashe JS, Hoffman BL, Spong CY, Casey BM. Williams Obstetrics. 26th ed. New York, NY, USA: McGraw-Hill Medical; 2022.
-
- Bromley B, Lieberman E, Shipp TD, Benacerraf BR. The genetic sonogram: a method of risk assessment for down syndrome in the second trimester. J Ultrasound Med. 2002;21(10):1087–1096. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources