Altered purinergic P2X7 and A2B receptors signaling limits macrophage-mediated host defense in schistosomiasis
- PMID: 38442854
- PMCID: PMC11550761
- DOI: 10.1016/j.bj.2024.100713
Altered purinergic P2X7 and A2B receptors signaling limits macrophage-mediated host defense in schistosomiasis
Abstract
Background: The occurrence of co-infections during schistosomiasis, a neglected tropical disease, with other parasites have been reported suggesting an impaired host immune defense. Macrophage purinergic P2X7 receptor (P2X7R) plays an important role against intracellular pathogens. Therefore, we investigated the P2X7R-mediated phagocytosis and killing capacity of Leishmania amazonensis by macrophages during schistosomiasis in vitro and in vivo.
Methods: Swiss and C57BL/6 (Wild type) and P2X7R-/- were randomized in two groups: control (uninfected) and Schistosoma mansoni-infected. Alternatively, control Swiss and S. mansoni-infected mice were also infected with L. amazonensis.
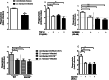
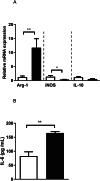
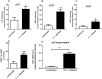
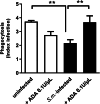
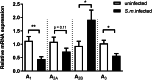
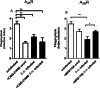
Results: The pre-treatment of control macrophages with the P2X7R antagonist (A74003) or TGF-β reduced the phagocytosis index, mimicking the phenotype of cells from S. mansoni-infected mice and P2X7R-/- mice. Apyrase also reduced the phagocytosis index in the control group corroborating the role of ATP to macrophage activation. Moreover, l-arginine-nitric oxide pathway was compromised during schistosomiasis, which could explain the reduced killing capacity in response to ATP in vitro and in vivo. We found an increased extracellular nucleotide (ATP, ADP and AMP) hydrolysis along with an increased frequency of F4/80+ CD39+ macrophages from the S. mansoni-infected group. Moreover, the content of adenosine in the cell supernatant was higher in the S. mansoni-infected group in relation to controls. Schistosomiasis also increased the expression of macrophage adenosine A2BR. In good accordance, both ADA and the selective A2BR antagonist restored the phagocytosis index of macrophages from S. mansoni-infected group.
Conclusions: Altogether, the altered P2X7R and A2BR signaling limits the role of macrophages to host defense against L. amazonensis during schistosomiasis, potentially contributing to the pathophysiology and clinically relevant co-infections.
Keywords: Adenosine receptor; Host defense; Leishmaniasis; Macrophages; P2X7 receptor; Schistosomiasis.
© 2024 The Authors. Published by Elsevier B.V. on behalf of Chang Gung University. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Conflict of interest statement
Declaration of competing interest none.
Figures



Similar articles
-
Macrophage P2X7 receptor function is reduced during schistosomiasis: putative role of TGF- β1.Mediators Inflamm. 2014;2014:134974. doi: 10.1155/2014/134974. Epub 2014 Aug 24. Mediators Inflamm. 2014. PMID: 25276050 Free PMC article.
-
CD39 limits P2X7 receptor inflammatory signaling and attenuates sepsis-induced liver injury.J Hepatol. 2017 Oct;67(4):716-726. doi: 10.1016/j.jhep.2017.05.021. Epub 2017 May 26. J Hepatol. 2017. PMID: 28554875 Free PMC article.
-
Non-canonical NLRP3 inflammasome activation and IL-1β signaling are necessary to L. amazonensis control mediated by P2X7 receptor and leukotriene B4.PLoS Pathog. 2019 Jun 24;15(6):e1007887. doi: 10.1371/journal.ppat.1007887. eCollection 2019 Jun. PLoS Pathog. 2019. PMID: 31233552 Free PMC article.
-
[ATP-P2X7R signalling pathway and its effects in parasitic diseases].Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2017 Jul 24;29(4):526-529. doi: 10.16250/j.32.1374.2016250. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2017. PMID: 29508600 Review. Chinese.
-
Characterizing the presence and sensitivity of the P2X7 receptor in different compartments of the gut.J Innate Immun. 2012;4(5-6):529-41. doi: 10.1159/000336628. Epub 2012 Apr 13. J Innate Immun. 2012. PMID: 22508425 Free PMC article. Review.
References
-
- Dunne DW, Cooke A. A worm’s eye view of the immune system: consequences for evolution of human autoimmune disease. Nature Rev Immunol. 2005;5(5):420–426. - PubMed
-
- Silva CLM., Morel N, Lenzi HL, Noël F. Increased reactivity to 5-hydroxytryptamine of portal veins from mice infected with Schistosoma mansoni. Comp Biochem Physiol Mol Integr Physiol. 1998;120(3):417–423. - PubMed
-
- Brunet RL, Beall M, Dunne DW, Pearce EJ. Nitric oxide and the Th2 response combine to prevent severe hepatic damage during Schistosoma mansoni infection. J Immunol. 1999;163(9):4976–4984. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

