Extracellular vesicles as a promising source of lipid biomarkers for breast cancer detection in blood plasma
- PMID: 38443328
- PMCID: PMC10914699
- DOI: 10.1002/jev2.12419
Extracellular vesicles as a promising source of lipid biomarkers for breast cancer detection in blood plasma
Abstract
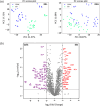
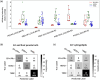
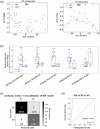
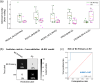
Extracellular vesicles (EVs), including exosomes and microvesicles, mediate intercellular communication in cancer, from development to metastasis. EV-based liquid biopsy is a promising strategy for cancer diagnosis as EVs can be found in cancer patients' body fluids. In this study, the lipid composition of breast cancer-derived EVs was studied as well as the potential of blood plasma EVs for the identification of lipid biomarkers for breast cancer detection. Initially, an untargeted lipidomic analysis was carried out for a panel of cancerous and non-cancerous mammary epithelial cells and their secreted EVs. We found that breast cancer-derived EVs are enriched in sphingolipids and glycerophospholipids compared to their parental cells. The initial in vitro study showed that EVs and their parental cells can be correctly classified (100% accuracy) between cancerous and non-cancerous, as well as into their respective breast cancer subtypes, based on their lipid composition. Subsequently, an untargeted lipidomic analysis was carried out for blood plasma EVs from women diagnosed with breast cancer (primary or progressive metastatic breast cancer) as well as healthy women. Correspondingly, when blood plasma EVs were analysed, breast cancer patients and healthy women were correctly classified with an overall accuracy of 93.1%, based on the EVs' lipid composition. Similarly, the analysis of patients with primary breast cancer and healthy women showed an overall accuracy of 95% for their correct classification. Furthermore, primary and metastatic breast cancers were correctly classified with an overall accuracy of 89.5%. This reveals that the blood plasma EVs' lipids may be a promising source of biomarkers for detection of breast cancer. Additionally, this study demonstrates the usefulness of untargeted lipidomics in the study of EV lipid composition and EV-associated biomarker discovery studies. This is a proof-of-concept study and a starting point for further analysis on the identification of EV-based biomarkers for breast cancer.
Keywords: breast cancer; extracellular vesicles; lipidomics; liquid biopsy; mass spectrometry.
© 2024 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
E.D. acknowledges support from Merck KGaA, Darmstadt, Germany, through the STRATiGRAD PhD program at Imperial College London. A.N. and M.M.S. acknowledge support from the GlaxoSmithKline Engineered Medicines Laboratory. M.M.S. acknowledges support from the Rosetrees Trust. These organisations did not play any role in the study design, data collection/analysis, manuscript preparation or publication. This work is the subject of an Imperial College Invention Disclosure application.
Figures


References
-
- Alimirzaie, S. , Bagherzadeh, M. , & Akbari, M. R. (2019). Liquid biopsy in breast cancer: A comprehensive review. Clinical Genetics, 95(6), 643–660. - PubMed
-
- Arnold, M. , Morgan, E. , Rumgay, H. , Mafra, A. , Singh, D. , Laversanne, M. , Vignat, J. , Gralow, J. R. , Cardoso, F. , Siesling, S. , & Soerjomataram, I. (2022). Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast (Edinburgh, Scotland), 66, 15–23. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

