Bevacizumab, olaparib, and durvalumab in patients with relapsed ovarian cancer: a phase II clinical trial from the GINECO group
- PMID: 38443333
- PMCID: PMC10914754
- DOI: 10.1038/s41467-024-45974-w
Bevacizumab, olaparib, and durvalumab in patients with relapsed ovarian cancer: a phase II clinical trial from the GINECO group
Erratum in
-
Author Correction: Bevacizumab, olaparib, and durvalumab in patients with relapsed ovarian cancer: a phase II clinical trial from the GINECO group.Nat Commun. 2024 Jun 4;15(1):4753. doi: 10.1038/s41467-024-48915-9. Nat Commun. 2024. PMID: 38834579 Free PMC article. No abstract available.
Abstract
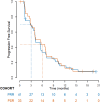
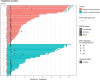
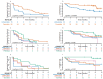
Most patients with advanced ovarian cancer (AOC) ultimately relapse after platinum-based chemotherapy. Combining bevacizumab, olaparib, and durvalumab likely drives synergistic activity. This open-label phase 2 study (NCT04015739) aimed to assess activity and safety of this triple combination in female patients with relapsed high-grade AOC following prior platinum-based therapy. Patients were treated with olaparib (300 mg orally, twice daily), the bevacizumab biosimilar FKB238 (15 mg/kg intravenously, once-every-3-weeks), and durvalumab (1.12 g intravenously, once-every-3-weeks) in nine French centers. The primary endpoint was the non-progression rate at 3 months for platinum-resistant relapse or 6 months for platinum-sensitive relapse per RECIST 1.1 and irRECIST. Secondary endpoints were CA-125 decline with CA-125 ELIMination rate constant K (KELIM-B) per CA-125 longitudinal kinetics over 100 days, progression free survival and overall survival, tumor response, and safety. Non-progression rates were 69.8% (90%CI 55.9%-80.0%) at 3 months for platinum-resistant relapse patients (N = 41), meeting the prespecified endpoint, and 43.8% (90%CI 29.0%-57.4%) at 6 months for platinum-sensitive relapse (N = 33), not meeting the prespecified endpoint. Median progression-free survival was 4.1 months (95%CI 3.5-5.9) and 4.9 months (95%CI 2.9-7.0) respectively. Favorable KELIM-B was associated with better survival. No toxic deaths or major safety signals were observed. Here we show that further investigation of this triple combination may be considered in AOC patients with platinum-resistant relapse.
© 2024. The Author(s).
Conflict of interest statement
Figures
References
-
- Coleman RL, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779–791. doi: 10.1016/S1470-2045(17)30279-6. - DOI - PMC - PubMed
-
- Aghajanian C, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. 2012;30:2039–2045. doi: 10.1200/JCO.2012.42.0505. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

