Brain regulates weight bearing bone through PGE2 skeletal interoception: implication of ankle osteoarthritis and pain
- PMID: 38443372
- PMCID: PMC10914853
- DOI: 10.1038/s41413-024-00316-w
Brain regulates weight bearing bone through PGE2 skeletal interoception: implication of ankle osteoarthritis and pain
Abstract
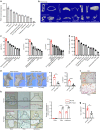
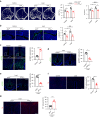
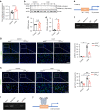
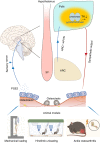
Bone is a mechanosensitive tissue and undergoes constant remodeling to adapt to the mechanical loading environment. However, it is unclear whether the signals of bone cells in response to mechanical stress are processed and interpreted in the brain. In this study, we found that the hypothalamus of the brain regulates bone remodeling and structure by perceiving bone prostaglandin E2 (PGE2) concentration in response to mechanical loading. Bone PGE2 levels are in proportion to their weight bearing. When weight bearing changes in the tail-suspension mice, the PGE2 concentrations in bones change in line with their weight bearing changes. Deletion of cyclooxygenase-2 (COX2) in the osteoblast lineage cells or knockout of receptor 4 (EP4) in sensory nerve blunts bone formation in response to mechanical loading. Moreover, knockout of TrkA in sensory nerve also significantly reduces mechanical load-induced bone formation. Moreover, mechanical loading induces cAMP-response element binding protein (CREB) phosphorylation in the hypothalamic arcuate nucleus (ARC) to inhibit sympathetic tyrosine hydroxylase (TH) expression in the paraventricular nucleus (PVN) for osteogenesis. Finally, we show that elevated PGE2 is associated with ankle osteoarthritis (AOA) and pain. Together, our data demonstrate that in response to mechanical loading, skeletal interoception occurs in the form of hypothalamic processing of PGE2-driven peripheral signaling to maintain physiologic bone homeostasis, while chronically elevated PGE2 can be sensed as pain during AOA and implication of potential treatment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Update of
-
Mechanical loading-induced change of bone homeostasis is mediated by PGE2-driven hypothalamic interoception.Res Sq [Preprint]. 2023 Sep 15:rs.3.rs-3325498. doi: 10.21203/rs.3.rs-3325498/v1. Res Sq. 2023. Update in: Bone Res. 2024 Mar 5;12(1):16. doi: 10.1038/s41413-024-00316-w. PMID: 37790467 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

