DJ-1 protects proteins from acylation by catalyzing the hydrolysis of highly reactive cyclic 3-phosphoglyceric anhydride
- PMID: 38443379
- PMCID: PMC10915168
- DOI: 10.1038/s41467-024-46391-9
DJ-1 protects proteins from acylation by catalyzing the hydrolysis of highly reactive cyclic 3-phosphoglyceric anhydride
Abstract
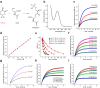
Mutations in the human PARK7 gene that encodes protein DJ-1 lead to familial Parkinsonism due to loss of dopaminergic neurons. However, the molecular function of DJ-1 underpinning its cytoprotective effects are unclear. Recently, DJ-1 has been shown to prevent acylation of amino groups of proteins and metabolites by 1,3-bisphosphoglycerate. This acylation is indirect and thought to proceed via the formation of an unstable intermediate, presumably a cyclic 3-phosphoglyceric anhydride (cPGA). Several lines of evidence indicate that DJ-1 destroys cPGA, however this enzymatic activity has not been directly demonstrated. Here, we report simple and effective procedures for synthesis and quantitation of cPGA and present a comprehensive characterization of this highly reactive acylating electrophile. We demonstrate that DJ-1 is an efficient cPGA hydrolase with kcat/Km = 5.9 × 106 M-1s-1. Experiments with DJ-1-null cells reveal that DJ-1 protects against accumulation of 3-phosphoglyceroyl-lysine residues in proteins. Our results establish a definitive cytoprotective function for DJ-1 that uses catalytic hydrolysis of cPGA to mitigate the damage from this glycolytic byproduct.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
cPGA hydrolase assay of DJ-1 in crude cell lysates: Implications for sensing of oxidative stress.Anal Biochem. 2024 Nov;694:115631. doi: 10.1016/j.ab.2024.115631. Epub 2024 Jul 30. Anal Biochem. 2024. PMID: 39084336
-
The apparent deglycase activity of DJ-1 results from the conversion of free methylglyoxal present in fast equilibrium with hemithioacetals and hemiaminals.J Biol Chem. 2019 Dec 6;294(49):18863-18872. doi: 10.1074/jbc.RA119.011237. Epub 2019 Oct 24. J Biol Chem. 2019. PMID: 31653696 Free PMC article.
-
Parkinson's disease protein PARK7 prevents metabolite and protein damage caused by a glycolytic metabolite.Proc Natl Acad Sci U S A. 2022 Jan 25;119(4):e2111338119. doi: 10.1073/pnas.2111338119. Proc Natl Acad Sci U S A. 2022. PMID: 35046029 Free PMC article.
-
Enhanced activity of glycolytic enzymes in Drosophila and human cell models of Parkinson's disease based on DJ-1 deficiency.Free Radic Biol Med. 2020 Oct;158:137-148. doi: 10.1016/j.freeradbiomed.2020.06.036. Epub 2020 Jul 26. Free Radic Biol Med. 2020. PMID: 32726690
-
DJ-1 and prevention of oxidative stress in Parkinson's disease and other age-related disorders.Free Radic Biol Med. 2009 Nov 15;47(10):1354-61. doi: 10.1016/j.freeradbiomed.2009.08.003. Epub 2009 Aug 14. Free Radic Biol Med. 2009. PMID: 19686841 Review.
Cited by
-
The origin of esterase activity of Parkinson's disease causative factor DJ-1 implied by evolutionary trace analysis of its prokaryotic homolog HchA.J Biol Chem. 2024 Jul;300(7):107476. doi: 10.1016/j.jbc.2024.107476. Epub 2024 Jun 13. J Biol Chem. 2024. PMID: 38879013 Free PMC article.
-
Reactivity-based metabolomics reveal cysteine has glyoxalase 1-like and glyoxalase 2-like activities.Nat Chem Biol. 2025 May 28. doi: 10.1038/s41589-025-01909-0. Online ahead of print. Nat Chem Biol. 2025. PMID: 40437135
-
Development and use of DJ-1 affinity microcolumns to screen and study small drug candidates for Parkinson's disease.Anal Chim Acta. 2025 Jan 22;1336:343520. doi: 10.1016/j.aca.2024.343520. Epub 2024 Dec 2. Anal Chim Acta. 2025. PMID: 39788673
-
Metabolic Dysregulation in Parkinson's Disease: Non-Oxidative Phosphorylation and Its Role in Brain Energy Metabolism.Aging Dis. 2025 Jun 22;16(5):2721-2738. doi: 10.14336/AD.2025.0619. Aging Dis. 2025. PMID: 40681352 Free PMC article. Review.
-
The reaction mechanism for glycolysis side product degradation by Parkinson's disease-linked DJ-1.J Cell Biol. 2025 Aug 4;224(8):e202411078. doi: 10.1083/jcb.202411078. Epub 2025 Jun 4. J Cell Biol. 2025. PMID: 40464736 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

