DJ-1 protects proteins from acylation by catalyzing the hydrolysis of highly reactive cyclic 3-phosphoglyceric anhydride
- PMID: 38443379
- PMCID: PMC10915168
- DOI: 10.1038/s41467-024-46391-9
DJ-1 protects proteins from acylation by catalyzing the hydrolysis of highly reactive cyclic 3-phosphoglyceric anhydride
Abstract
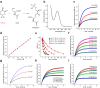
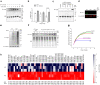
Mutations in the human PARK7 gene that encodes protein DJ-1 lead to familial Parkinsonism due to loss of dopaminergic neurons. However, the molecular function of DJ-1 underpinning its cytoprotective effects are unclear. Recently, DJ-1 has been shown to prevent acylation of amino groups of proteins and metabolites by 1,3-bisphosphoglycerate. This acylation is indirect and thought to proceed via the formation of an unstable intermediate, presumably a cyclic 3-phosphoglyceric anhydride (cPGA). Several lines of evidence indicate that DJ-1 destroys cPGA, however this enzymatic activity has not been directly demonstrated. Here, we report simple and effective procedures for synthesis and quantitation of cPGA and present a comprehensive characterization of this highly reactive acylating electrophile. We demonstrate that DJ-1 is an efficient cPGA hydrolase with kcat/Km = 5.9 × 106 M-1s-1. Experiments with DJ-1-null cells reveal that DJ-1 protects against accumulation of 3-phosphoglyceroyl-lysine residues in proteins. Our results establish a definitive cytoprotective function for DJ-1 that uses catalytic hydrolysis of cPGA to mitigate the damage from this glycolytic byproduct.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

