Synergistic antitumor activity between HER2 antibody-drug conjugate and chemotherapy for treating advanced colorectal cancer
- PMID: 38443386
- PMCID: PMC10914798
- DOI: 10.1038/s41419-024-06572-2
Synergistic antitumor activity between HER2 antibody-drug conjugate and chemotherapy for treating advanced colorectal cancer
Abstract
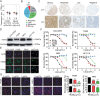
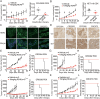
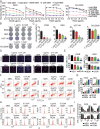
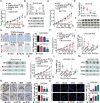
Colorectal cancer (CRC) is the third most common cancer associated with a poor prognosis. Effective targeted therapy alone or in combination for treating advanced CRC remains to be a major clinical challenge. Here, we propose the therapeutic efficacy and molecular mechanism underlying RC48, a FDA-approved anti-HER2 antibody conjugate via a cleavable linker to the microtubule inhibitor monomethyl auristatin E (MMAE), either alone or in combination with gemcitabine (GEM) in various models of HER2-positive advanced CRC. Our findings demonstrated that HER2 was widely expressed and located on the plasma membrane of CRC patient specimens, PDX xenograft tumors and cell lines. It confirmed that RC48 alone significantly targeted and eradicated HER2 positive CRC tumor in these models. Moreover, we screened a panel of FDA-approved first-line chemotherapy drugs in vitro. We found that GEM exhibited stronger antiproliferative activity compared to the other first-line anti-cancer agents. Furthermore, combination therapy of RC48 and GEM significantly showed synergetic antitumor activity in vitro and in vivo. To gain further mechanistic insights into the combination therapy, we performed RNA-seq analysis. The results revealed that combination treatment of RC48 and GEM regulated multiple signaling pathways, such as PI3K-AKT, MAPK, p53, Foxo, apoptosis, cell cycle and cell senescence, etc., to exert its antitumor activity in CRC cells. Collectively, these preclinical findings demonstrated that RC48 alone or combinational therapy exerted promising antitumor activity, and meriting the preclinical framework for combinational therapy of anti-HER2 drug conjugate drug and chemotherapy drugs for HER2-positive patients with advanced CRC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Combined inhibition of HER2 and VEGFR synergistically improves therapeutic efficacy via PI3K-AKT pathway in advanced ovarian cancer.J Exp Clin Cancer Res. 2024 Feb 26;43(1):56. doi: 10.1186/s13046-024-02981-5. J Exp Clin Cancer Res. 2024. PMID: 38403634 Free PMC article.
-
From AVATAR Mice to Patients: RC48-ADC Exerted Promising Efficacy in Advanced Gastric Cancer With HER2 Expression.Front Pharmacol. 2022 Jan 5;12:757994. doi: 10.3389/fphar.2021.757994. eCollection 2021. Front Pharmacol. 2022. PMID: 35069192 Free PMC article.
-
A HER2-targeted Antibody-Drug Conjugate, RC48-ADC, Exerted Promising Antitumor Efficacy and Safety with Intravesical Instillation in Preclinical Models of Bladder Cancer.Adv Sci (Weinh). 2023 Nov;10(32):e2302377. doi: 10.1002/advs.202302377. Epub 2023 Oct 12. Adv Sci (Weinh). 2023. PMID: 37824205 Free PMC article.
-
Disitamab vedotin, a novel HER2-directed antibody-drug conjugate in gastric cancer and other solid tumors.Drugs Today (Barc). 2022 Oct;58(10):491-507. doi: 10.1358/dot.2022.58.10.3408812. Drugs Today (Barc). 2022. PMID: 36305543 Review.
-
mTOR inhibitor introduce disitamab vedotin (RC48-ADC) rechallenge microtubule-chemotherapy resistance in HER2-low MBC patients with PI3K mutation.Front Oncol. 2024 Jan 25;14:1312634. doi: 10.3389/fonc.2024.1312634. eCollection 2024. Front Oncol. 2024. PMID: 38344201 Free PMC article. Review.
Cited by
-
Anti-tumour effects of lapatinib on HER2-positive canine prostatic carcinoma cell lines.Open Vet J. 2024 May;14(5):1259-1268. doi: 10.5455/OVJ.2024.v14.i5.21. Epub 2024 May 31. Open Vet J. 2024. PMID: 38938437 Free PMC article.
-
Colorectal Cancer: Current and Future Therapeutic Approaches and Related Technologies Addressing Multidrug Strategies Against Multiple Level Resistance Mechanisms.Int J Mol Sci. 2025 Feb 4;26(3):1313. doi: 10.3390/ijms26031313. Int J Mol Sci. 2025. PMID: 39941081 Free PMC article. Review.
-
Precision oncolytic viral therapy in colorectal cancer: Genetic targeting and immune modulation for personalized treatment (Review).Int J Mol Med. 2025 Jul;56(1):104. doi: 10.3892/ijmm.2025.5545. Epub 2025 May 9. Int J Mol Med. 2025. PMID: 40342021 Free PMC article. Review.
-
Copper-based metal-organic frameworks for antitumor application.J Nanobiotechnology. 2025 Feb 22;23(1):135. doi: 10.1186/s12951-025-03220-5. J Nanobiotechnology. 2025. PMID: 39987136 Free PMC article. Review.
-
Investigation of the cytotoxic effects and mechanisms of the SLC39A6-targeting ADC drug BRY812 in CRC.Sci Rep. 2025 May 25;15(1):18275. doi: 10.1038/s41598-025-03713-1. Sci Rep. 2025. PMID: 40414981 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

