FacZ is a GpsB-interacting protein that prevents aberrant division-site placement in Staphylococcus aureus
- PMID: 38443581
- PMCID: PMC10914604
- DOI: 10.1038/s41564-024-01607-y
FacZ is a GpsB-interacting protein that prevents aberrant division-site placement in Staphylococcus aureus
Abstract
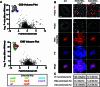
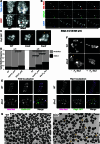
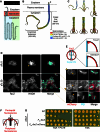
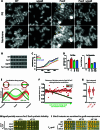
Staphylococcus aureus is a Gram-positive pathogen responsible for antibiotic-resistant infections. To identify vulnerabilities in cell envelope biogenesis that may overcome resistance, we enriched for S. aureus transposon mutants with defects in cell surface integrity or cell division by sorting for cells that stain with propidium iodide or have increased light-scattering properties, respectively. Transposon sequencing of the sorted populations identified more than 20 previously uncharacterized factors impacting these processes. Cells inactivated for one of these proteins, factor preventing extra Z-rings (FacZ, SAOUHSC_01855), showed aberrant membrane invaginations and multiple FtsZ cytokinetic rings. These phenotypes were suppressed in mutants lacking the conserved cell-division protein GpsB, which forms an interaction hub bridging envelope biogenesis factors with the cytokinetic ring in S. aureus. FacZ was found to interact directly with GpsB in vitro and in vivo. We therefore propose that FacZ is an envelope biogenesis factor that antagonizes GpsB function to prevent aberrant division events in S. aureus.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Update of
-
Identification of FacZ as a division site placement factor in Staphylococcus aureus.bioRxiv [Preprint]. 2023 Apr 24:2023.04.24.538170. doi: 10.1101/2023.04.24.538170. bioRxiv. 2023. Update in: Nat Microbiol. 2024 Mar;9(3):801-813. doi: 10.1038/s41564-024-01607-y. PMID: 37162900 Free PMC article. Updated. Preprint.
References
-
- Koch AL. Growth and form of the bacterial cell wall. Am. Sci. 1990;78:327–341.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

