RNA aptamer reveals nuclear TDP-43 pathology is an early aggregation event that coincides with STMN-2 cryptic splicing and precedes clinical manifestation in ALS
- PMID: 38443601
- PMCID: PMC10914926
- DOI: 10.1007/s00401-024-02705-1
RNA aptamer reveals nuclear TDP-43 pathology is an early aggregation event that coincides with STMN-2 cryptic splicing and precedes clinical manifestation in ALS
Abstract
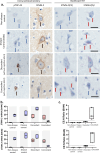
TDP-43 is an aggregation-prone protein which accumulates in the hallmark pathological inclusions of amyotrophic lateral sclerosis (ALS). However, the analysis of deeply phenotyped human post-mortem samples has shown that TDP-43 aggregation, revealed by standard antibody methods, correlates poorly with symptom manifestation. Recent identification of cryptic-splicing events, such as the detection of Stathmin-2 (STMN-2) cryptic exons, are providing evidence implicating TDP-43 loss-of-function as a potential driving pathomechanism but the temporal nature of TDP-43 loss and its relation to the disease process and clinical phenotype is not known. To address these outstanding questions, we used a novel RNA aptamer, TDP-43APT, to detect TDP-43 pathology and used single molecule in situ hybridization to sensitively reveal TDP-43 loss-of-function and applied these in a deeply phenotyped human post-mortem tissue cohort. We demonstrate that TDP-43APT identifies pathological TDP-43, detecting aggregation events that cannot be detected by classical antibody stains. We show that nuclear TDP-43 pathology is an early event, occurring prior to cytoplasmic accumulation and is associated with loss-of-function measured by coincident STMN-2 cryptic splicing pathology. Crucially, we show that these pathological features of TDP-43 loss-of-function precede the clinical inflection point and are not required for region specific clinical manifestation. Furthermore, we demonstrate that gain-of-function in the form of extensive cytoplasmic accumulation, but not loss-of-function, is the primary molecular correlate of clinical manifestation. Taken together, our findings demonstrate implications for early diagnostics as the presence of STMN-2 cryptic exons and early TDP-43 aggregation events could be detected prior to symptom onset, holding promise for early intervention in ALS.
Keywords: Stathmin-2; Amyotrophic lateral sclerosis; Cognition; Cryptic splicing; Loss-of-function; Neuropathology; RNA aptamer; TDP-43.
© 2024. The Author(s).
Conflict of interest statement
GGT, EZ, AP, MH are listed as inventors on a patent relating to the use of TDP-43 binding RNA aptamers: “WO2023217742—TDP-43-BINDING SINGLE-STRANDED APTAMERS AND USES THEREOF” (PCT/EP2023/062203). The authors declare no other conflicts of interest.
Figures



References
-
- Altman T, Ionescu A, Ibraheem A, Priesmann D, Gradus-Pery T, Farberov L, et al. Axonal TDP-43 condensates drive neuromuscular junction disruption through inhibition of local synthesis of nuclear encoded mitochondrial proteins. Nat Commun. 2021;12:6914. doi: 10.1038/s41467-021-27221-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

