Adjuvant Chemoradiation in Resected Biliary Adenocarcinoma: Evaluation of SWOG S0809 with a Large National Database
- PMID: 38443700
- PMCID: PMC11236922
- DOI: 10.1245/s10434-024-15117-y
Adjuvant Chemoradiation in Resected Biliary Adenocarcinoma: Evaluation of SWOG S0809 with a Large National Database
Abstract
Background: There is a paucity of evidence supporting the use of adjuvant radiation therapy in resected biliary cancer. Supporting evidence for use comes mainly from the small SWOG S0809 trial, which demonstrated an overall median survival of 35 months. We aimed to use a large national database to evaluate the use of adjuvant chemoradiation in resected extrahepatic bile duct and gallbladder cancer.
Methods: Using the National Cancer Database, we selected patients from 2004 to 2017 with pT2-4, pN0-1, M0 extrahepatic bile duct or gallbladder adenocarcinoma with either R0 or R1 resection margins, and examined factors associated with overall survival (OS). We examined OS in a cohort of patients mimicking the SWOG S0809 protocol as a large validation cohort. Lastly, we compared patients who received chemotherapy only with patients who received adjuvant chemotherapy and radiation using entropy balancing propensity score matching.
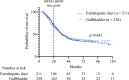
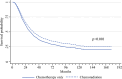
Results: Overall, 4997 patients with gallbladder or extrahepatic bile duct adenocarcinoma with available survival information meeting the SWOG S0809 criteria were selected, 469 of whom received both adjuvant chemotherapy and radiotherapy. Median OS in patients undergoing chemoradiation was 36.9 months, and was not different between primary sites (p = 0.841). In a propensity score matched cohort, receipt of adjuvant chemoradiation had a survival benefit compared with adjuvant chemotherapy only (hazard ratio 0.86, 95% confidence interval 0.77-0.95; p = 0.004).
Conclusion: Using a large national database, we support the findings of SWOG S0809 with a similar median OS in patients receiving chemoradiation. These data further support the consideration of adjuvant multimodal therapy in resected biliary cancers.
Keywords: Bile duct cancer; Chemoradiation; Cholangiocarcinoma; Gallbladder cancer; SWOG 0809.
© 2024. The Author(s).
Figures

References
-
- Jarnagin WR, Ruo L, Little SA, Klimstra D, D’Angelica M, DeMatteo RP, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98(8):1689–1700. doi: 10.1002/cncr.11699. - DOI - PubMed
-
- Nakachi K, Ikeda M, Konishi M, Nomura S, Katayama H, Kataoka T, et al. Adjuvant S-1 compared with observation in resected biliary tract cancer (JCOG1202, ASCOT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet. 2023;401(10372):195–203. doi: 10.1016/S0140-6736(22)02038-4. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

