tRigon: an R package and Shiny App for integrative (path-)omics data analysis
- PMID: 38443821
- PMCID: PMC10916305
- DOI: 10.1186/s12859-024-05721-w
tRigon: an R package and Shiny App for integrative (path-)omics data analysis
Abstract
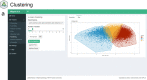
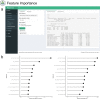
Background: Pathomics facilitates automated, reproducible and precise histopathology analysis and morphological phenotyping. Similar to molecular omics, pathomics datasets are high-dimensional, but also face large outlier variability and inherent data missingness, making quick and comprehensible data analysis challenging. To facilitate pathomics data analysis and interpretation as well as support a broad implementation we developed tRigon (Toolbox foR InteGrative (path-)Omics data aNalysis), a Shiny application for fast, comprehensive and reproducible pathomics analysis.
Results: tRigon is available via the CRAN repository ( https://cran.r-project.org/web/packages/tRigon ) with its source code available on GitLab ( https://git-ce.rwth-aachen.de/labooratory-ai/trigon ). The tRigon package can be installed locally and its application can be executed from the R console via the command 'tRigon::run_tRigon()'. Alternatively, the application is hosted online and can be accessed at https://labooratory.shinyapps.io/tRigon . We show fast computation of small, medium and large datasets in a low- and high-performance hardware setting, indicating broad applicability of tRigon.
Conclusions: tRigon allows researchers without coding abilities to perform exploratory feature analyses of pathomics and non-pathomics datasets on their own using a variety of hardware.
Keywords: Data exploration; Pathomics; Statistics; User interface.
© 2024. The Author(s).
Conflict of interest statement
The authors declare they have no competing interests.
Figures





Similar articles
-
RawHummus: an R Shiny app for automated raw data quality control in metabolomics.Bioinformatics. 2022 Mar 28;38(7):2072-2074. doi: 10.1093/bioinformatics/btac040. Bioinformatics. 2022. PMID: 35080628
-
CCWeights: an R package and web application for automated evaluation and selection of weighting factors for accurate quantification using linear calibration curve.Bioinform Adv. 2021 Oct 28;1(1):vbab029. doi: 10.1093/bioadv/vbab029. eCollection 2021. Bioinform Adv. 2021. PMID: 36700106 Free PMC article.
-
sendigR: an R package to leverage the value of CDISC SEND datasets for cross-study analysis.Front Toxicol. 2024 Jul 15;6:1392686. doi: 10.3389/ftox.2024.1392686. eCollection 2024. Front Toxicol. 2024. PMID: 39077556 Free PMC article.
-
mvlearnR and Shiny App for multiview learning.Bioinform Adv. 2024 Jan 16;4(1):vbae005. doi: 10.1093/bioadv/vbae005. eCollection 2024. Bioinform Adv. 2024. PMID: 38304121 Free PMC article.
-
CNVScope: Visually Exploring Copy Number Aberrations in Cancer Genomes.Cancer Inform. 2019 Dec 2;18:1176935119890290. doi: 10.1177/1176935119890290. eCollection 2019. Cancer Inform. 2019. PMID: 31832011 Free PMC article. Review.
Cited by
-
Decoding pathology: the role of computational pathology in research and diagnostics.Pflugers Arch. 2025 Apr;477(4):555-570. doi: 10.1007/s00424-024-03002-2. Epub 2024 Aug 3. Pflugers Arch. 2025. PMID: 39095655 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

