Toxicities, intensive care management, and outcome of chimeric antigen receptor T cells in adults: an update
- PMID: 38444031
- PMCID: PMC10916319
- DOI: 10.1186/s13054-024-04851-0
Toxicities, intensive care management, and outcome of chimeric antigen receptor T cells in adults: an update
Abstract
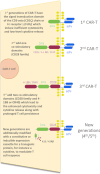
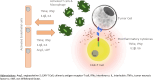
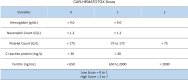
Background: Chimeric antigen receptor T cells are a promising new immunotherapy for haematological malignancies. Six CAR-T cells products are currently available for adult patients with refractory or relapsed high-grade B cell malignancies, but they are associated with severe life-threatening toxicities and side effects that may require admission to ICU.
Objective: The aim of this short pragmatic review is to synthesize for intensivists the knowledge on CAR-T cell therapy with emphasis on CAR-T cell-induced toxicities and ICU management of complications according to international recommendations, outcomes and future issues.
Keywords: CAR-T cell therapy; Haematological malignancies; Intensive care management; Toxicities.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

