Cardiac progenitor cell therapy: mechanisms of action
- PMID: 38444042
- PMCID: PMC10913616
- DOI: 10.1186/s13578-024-01211-x
Cardiac progenitor cell therapy: mechanisms of action
Abstract
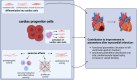
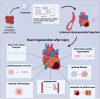
Heart failure (HF) is an end-stage of many cardiac diseases and one of the main causes of death worldwide. The current management of this disease remains suboptimal. The adult mammalian heart was considered a post-mitotic organ. However, several reports suggest that it may possess modest regenerative potential. Adult cardiac progenitor cells (CPCs), the main players in the cardiac regeneration, constitute, as it may seem, a heterogenous group of cells, which remain quiescent in physiological conditions and become activated after an injury, contributing to cardiomyocytes renewal. They can mediate their beneficial effects through direct differentiation into cardiac cells and activation of resident stem cells but majorly do so through paracrine release of factors. CPCs can secrete cytokines, chemokines, and growth factors as well as exosomes, rich in proteins, lipids and non-coding RNAs, such as miRNAs and YRNAs, which contribute to reparation of myocardium by promoting angiogenesis, cardioprotection, cardiomyogenesis, anti-fibrotic activity, and by immune modulation. Preclinical studies assessing cardiac progenitor cells and cardiac progenitor cells-derived exosomes on damaged myocardium show that administration of cardiac progenitor cells-derived exosomes can mimic effects of cell transplantation. Exosomes may become new promising therapeutic strategy for heart regeneration nevertheless there are still several limitations as to their use in the clinic. Key questions regarding their dosage, safety, specificity, pharmacokinetics, pharmacodynamics and route of administration remain outstanding. There are still gaps in the knowledge on basic biology of exosomes and filling them will bring as closer to translation into clinic.
Keywords: Adult cardiac progenitor cells; Cardiac regeneration; Exosomes; Non-coding RNAs.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Yancy CW, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the management of Heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines and the heart failure society of Amer. Circulation. 2016;134(13). 10.1161/CIR.0000000000000509. - PubMed
-
- Virani SS, et al. Heart Disease and Stroke Statistics—2023 update: a Report from the American Heart Association. Circulation. 2021;148(4). 10.1161/CIR.0000000000000950. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

