Distinct potassium channel types in brain capillary pericytes
- PMID: 38444160
- PMCID: PMC11309962
- DOI: 10.1016/j.bpj.2024.03.004
Distinct potassium channel types in brain capillary pericytes
Abstract
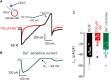
Capillaries, composed of electrically coupled endothelial cells and overlying pericytes, constitute the vast majority of blood vessels in the brain. The most arteriole-proximate three to four branches of the capillary bed are covered by α-actin-expressing, contractile pericytes. These mural cells have a distinctive morphology and express different markers compared with their smooth muscle cell (SMC) cousins but share similar excitation-coupling contraction machinery. Despite this similarity, pericytes are considerably more depolarized than SMCs at low intravascular pressures. We have recently shown that pericytes, such as SMCs, possess functional voltage-dependent Ca2+ channels and ATP-sensitive K+ channels. Here, we further investigate the complement of pericyte ion channels, focusing on members of the K+ channel superfamily. Using NG2-DsRed-transgenic mice and diverse configurations of the patch-clamp technique, we demonstrate that pericytes display robust inward-rectifier K+ currents that are primarily mediated by the Kir2 family, based on their unique biophysical characteristics and sensitivity to micromolar concentrations of Ba2+. Moreover, multiple lines of evidence, including characteristic kinetics, sensitivity to specific blockers, biophysical attributes, and distinctive single-channel properties, established the functional expression of two voltage-dependent K+ channels: KV1 and BKCa. Although these three types of channels are also present in SMCs, they exhibit distinctive current density and kinetics profiles in pericytes. Collectively, these findings underscore differences in the operation of shared molecular features between pericytes and SMCs and highlight the potential contribution of these three K+ ion channels in setting pericyte membrane potential, modulating capillary hemodynamics, and regulating cerebral blood flow.
Copyright © 2024 Biophysical Society. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
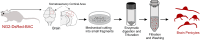




Comment in
-
Juggling potassium: A diverse set of K+ channels tune excitability of brain's capillary pericytes.Biophys J. 2024 Jul 16;123(14):1910-1911. doi: 10.1016/j.bpj.2024.03.037. Epub 2024 Mar 27. Biophys J. 2024. PMID: 38549373 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

