Diabetic Ketoacidosis at Onset of Type 1 Diabetes and Glycemic Outcomes with Closed-Loop Insulin Delivery
- PMID: 38444312
- PMCID: PMC10877390
- DOI: 10.1089/dia.2023.0307
Diabetic Ketoacidosis at Onset of Type 1 Diabetes and Glycemic Outcomes with Closed-Loop Insulin Delivery
Abstract
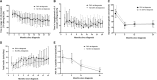
The presence of diabetic ketoacidosis (DKA) at diagnosis of type 1 diabetes (T1D) is associated with higher glycated hemoglobin levels over time. We evaluated whether hybrid-closed loop (HCL) therapy from onset of T1D could prevent the adverse impact of DKA at diagnosis on long-term glycemic outcomes. This was a posthoc analysis from 51 adolescents using HCL from diagnosis of T1D as part of the CLOuD trial (NCT02871089). We compared glycemic and insulin metrics between adolescents with (n = 17) and without (n = 34) DKA at diagnosis. Participants with and without DKA at diagnosis had similar time in target glucose range 3.9-10.0 mmol/L (70-180 mg/dL), time below range (<3.9 mmol/L, <70 mg/dL) and HbA1c at 6, 12, and 24 months. While insulin requirements at 6 months were higher in those with DKA at diagnosis, this was not statistically significant after adjusting for bodyweight. Residual C-peptide secretion was similar between groups. We conclude that HCL therapy may mitigate against the negative glycemic effects of DKA at T1D diagnosis.
Keywords: Artificial pancreas; Closed-loop systems; Diabetic ketoacidosis; Type 1 diabetes.
Conflict of interest statement
C.K.B. has received consulting fees from CamDiab and speaker honoraria from Ypsomed. J.W. reports receiving speaker honoraria from Ypsomed. M.E.W. reports patents related to closed-loop and being a consultant at CamDiab. S.H. serves as a member of Medtronic advisory board, is a director of Ask Diabetes Ltd providing training and research support in health care settings, and reports having received training honoraria from Medtronic and Sanofi and consulting fees for CamDiab. T.R. receives consultancy fees from Abbott Diabetes care and has received honoraria from NovoNordisk for delivering educational meetings. R.E.J.B. reports receiving speaker honoraria from Eli Lilly and Springer Healthcare, and reports sitting on the NovoNordisk UK Foundation Research Selection Committee on a voluntary basis. R.H. reports receiving speaker honoraria from Eli Lilly, Dexcom, and Novo Nordisk, receiving license and/or consultancy fees from BBraun and Abbott Diabetes Care; patents related to closed-loop, and being director at CamDiab. M.N., R.L., J.M.A., A.T., A.G., D.E., N.T., and F.M.C. declare no competing financial interests exist.
Figures
References
-
- National Paediatrics Diabets Audit, Annual report 2020–21: Care processes and outcomes. 2021. Available from: https://www.rcpch.ac.uk/resources/npda-annual-reports#downloadBox [Last accessed: April 25, 2023].
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
