Modulation of chronic obstructive pulmonary disease progression by antioxidant metabolites from Pediococcus pentosaceus: enhancing gut probiotics abundance and the tryptophan-melatonin pathway
- PMID: 38444395
- PMCID: PMC10936690
- DOI: 10.1080/19490976.2024.2320283
Modulation of chronic obstructive pulmonary disease progression by antioxidant metabolites from Pediococcus pentosaceus: enhancing gut probiotics abundance and the tryptophan-melatonin pathway
Abstract
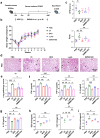
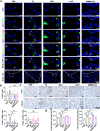
Chronic obstructive pulmonary disease (COPD), a condition primarily linked to oxidative stress, poses significant health burdens worldwide. Recent evidence has shed light on the association between the dysbiosis of gut microbiota and COPD, and their metabolites have emerged as potential modulators of disease progression through the intricate gut-lung axis. Here, we demonstrate the efficacy of oral administration of the probiotic Pediococcus pentosaceus SMM914 (SMM914) in delaying the progression of COPD by attenuating pulmonary oxidative stress. Specially, SMM914 induces a notable shift in the gut microbiota toward a community structure characterized by an augmented abundance of probiotics producing short-chain fatty acids and antioxidant metabolisms. Concurrently, SMM914 synthesizes L-tryptophanamide, 5-hydroxy-L-tryptophan, and 3-sulfino-L-alanine, thereby enhancing the tryptophan-melatonin pathway and elevating 6-hydroxymelatonin and hypotaurine in the lung environment. This modulation amplifies the secretion of endogenous anti-inflammatory factors, diminishes macrophage polarization toward the M1 phenotype, and ultimately mitigates the oxidative stress in mice with COPD. The demonstrated efficacy of the probiotic intervention, specifically with SMM914, not only highlights the modulation of intestine microbiota but also emphasizes the consequential impact on the intricate interplay between the gastrointestinal system and respiratory health.
Keywords: COPD; Microbial metabolites; gut-lung axis; oxidative stress.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures






References
-
- Nici L, Mammen MJ, Charbek E, Alexander PE, Au DH, Boyd CM, Criner GJ, Donaldson GC, Dreher M, Fan VS. et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American thoracic society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56–25. doi:10.1164/rccm.202003-0625ST. - DOI - PMC - PubMed
-
- Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med. 2022;10(5):447–458. doi:10.1016/S2213-2600(21)00511-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
