Pax2-cre-mediated deletion of Lgl1 causes abnormal development of the midbrain
- PMID: 38444736
- PMCID: PMC10912833
- DOI: 10.1016/j.bbrep.2024.101671
Pax2-cre-mediated deletion of Lgl1 causes abnormal development of the midbrain
Abstract
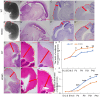
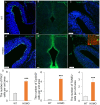
Lgl1 protein plays a critical role in neurodevelopment, including hippocampus, olfactory bulb, and Purkinje cell. However, the specific mechanism of LGL1 function in the midbrain remains elusive. In this study, we generated Lgl1 conditional knockout mice using Pax2-Cre, which is expressed in the midbrain, and examined the functions of Lgl1 in the midbrain. Histological analysis exhibited abnormal midbrain development characterized by enlarged ventricular aqueduct and thinning tectum cortex. Lgl1 deletion caused excessive proliferation and heightened apoptosis of neural progenitor cells in the tectum of LP cko mice. BrdU labeling studies demonstrated abnormal neuronal migration. Immunofluorescence analysis of Nestin demonstrated an irregular and clustered distribution of glial cell fibers, with the adhesion junction marker N-cadherin employed for immunofluorescent labeling, unveiling abnormal epithelial connections within the tectum of LP cko mice. The current findings suggest that the deletion of Lgl1 leads to the disruption of the expression pattern of N-cadherin, resulting in abnormal development of the midbrain.
Keywords: Lgl1; Midbrain; Migration; N-Cadherin.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Abnormal cerebellar development and Purkinje cell defects in Lgl1-Pax2 conditional knockout mice.Dev Biol. 2014 Nov 1;395(1):167-81. doi: 10.1016/j.ydbio.2014.07.007. Epub 2014 Jul 19. Dev Biol. 2014. PMID: 25050931
-
Loss of Lgl1 Disrupts the Radial Glial Fiber-guided Cortical Neuronal Migration and Causes Subcortical Band Heterotopia in Mice.Neuroscience. 2019 Feb 21;400:132-145. doi: 10.1016/j.neuroscience.2018.12.039. Epub 2018 Dec 28. Neuroscience. 2019. PMID: 30597194
-
Lgl1 Is Required for Olfaction and Development of Olfactory Bulb in Mice.PLoS One. 2016 Sep 7;11(9):e0162126. doi: 10.1371/journal.pone.0162126. eCollection 2016. PLoS One. 2016. PMID: 27603780 Free PMC article.
-
Genetic deletion of afadin causes hydrocephalus by destruction of adherens junctions in radial glial and ependymal cells in the midbrain.PLoS One. 2013 Nov 13;8(11):e80356. doi: 10.1371/journal.pone.0080356. eCollection 2013. PLoS One. 2013. PMID: 24236178 Free PMC article.
-
Lgl1 deficiency disrupts hippocampal development and impairs cognitive performance in mice.Genes Brain Behav. 2019 Nov;18(8):e12605. doi: 10.1111/gbb.12605. Epub 2019 Sep 3. Genes Brain Behav. 2019. PMID: 31415124
References
-
- Roberts B. Neuronal migration disorders. Radiol. Technol. 2018;89:279–295. - PubMed
-
- Poirier K., Saillour Y., Bahi-Buisson N., Jaglin X.H., Fallet-Bianco C., Nabbout R., Castelnau-Ptakhine L., Roubertie A., Attie-Bitach T., Desguerre I., Genevieve D., Barnerias C., Keren B., Lebrun N., Boddaert N., Encha-Razavi F., Chelly J. Mutations in the neuronal ss-tubulin subunit TUBB3 result in malformation of cortical development and neuronal migration defects. Hum. Mol. Genet. 2010;19:4462–4473. doi: 10.1093/hmg/ddq377. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

