Recurring SARS-CoV-2 variants: an update on post-pandemic, co-infections and immune response
- PMID: 38444741
- PMCID: PMC10911975
- DOI: 10.7150/ntno.91910
Recurring SARS-CoV-2 variants: an update on post-pandemic, co-infections and immune response
Abstract
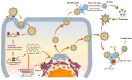
The post-pandemic era following the global spread of the SARS-CoV-2 virus has brought about persistent concerns regarding recurring coinfections. While significant strides in genome mapping, diagnostics, and vaccine development have controlled the pandemic and reduced fatalities, ongoing virus mutations necessitate a deeper exploration of the interplay between SARS-CoV-2 mutations and the host's immune response. Various vaccines, including RNA-based ones like Pfizer and Moderna, viral vector vaccines like Johnson & Johnson and AstraZeneca, and protein subunit vaccines like Novavax, have played critical roles in mitigating the impact of COVID-19. Understanding their strengths and limitations is crucial for tailoring future vaccines to specific variants and individual needs. The intricate relationship between SARS-CoV-2 mutations and the immune response remains a focus of intense research, providing insights into personalized treatment strategies and long-term effects like long-COVID. This article offers an overview of the post-pandemic landscape, highlighting emerging variants, summarizing vaccine platforms, and delving into immunological responses and the phenomenon of long-COVID. By presenting clinical findings, it aims to contribute to the ongoing understanding of COVID-19's progression in the aftermath of the pandemic.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
COVID-19 Pandemic and Vaccines Update on Challenges and Resolutions.Front Cell Infect Microbiol. 2021 Sep 10;11:690621. doi: 10.3389/fcimb.2021.690621. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34568087 Free PMC article. Review.
-
The COVID-19 pandemic: viral variants and vaccine efficacy.Crit Rev Clin Lab Sci. 2022 Jan;59(1):66-75. doi: 10.1080/10408363.2021.1979462. Epub 2021 Oct 1. Crit Rev Clin Lab Sci. 2022. PMID: 34598660 Review.
-
A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson.Hum Vaccin Immunother. 2022 Dec 31;18(1):2002083. doi: 10.1080/21645515.2021.2002083. Epub 2022 Feb 7. Hum Vaccin Immunother. 2022. PMID: 35130825 Free PMC article. Review.
-
Next-generation treatments: Immunotherapy and advanced therapies for COVID-19.Heliyon. 2024 Feb 19;10(5):e26423. doi: 10.1016/j.heliyon.2024.e26423. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38434363 Free PMC article. Review.
-
Application of Traditional Vaccine Development Strategies to SARS-CoV-2.mSystems. 2023 Apr 27;8(2):e0092722. doi: 10.1128/msystems.00927-22. Epub 2023 Mar 2. mSystems. 2023. PMID: 36861991 Free PMC article. Review.
Cited by
-
A novel viral RNA detection method based on the combined use of trans-acting ribozymes and HCR-FRET analyses.PLoS One. 2024 Sep 26;19(9):e0310171. doi: 10.1371/journal.pone.0310171. eCollection 2024. PLoS One. 2024. PMID: 39325749 Free PMC article.
-
The relationship between maternal emotional self-disclosure and children's anxiety in the post-COVID-19 era.BMC Psychiatry. 2025 Apr 16;25(1):381. doi: 10.1186/s12888-025-06810-7. BMC Psychiatry. 2025. PMID: 40241026 Free PMC article.
-
Into the Cauldron of the Variant Soup: Insights into the Molecular Epidemiology and Transition to Endemicity of SARS-CoV-2 in Cyprus (November 2022-February 2024).Viruses. 2024 Oct 29;16(11):1686. doi: 10.3390/v16111686. Viruses. 2024. PMID: 39599801 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

