Making the invisible visible: imaging techniques for assessing muscle mass and muscle quality in chronic kidney disease
- PMID: 38444750
- PMCID: PMC10913944
- DOI: 10.1093/ckj/sfae028
Making the invisible visible: imaging techniques for assessing muscle mass and muscle quality in chronic kidney disease
Abstract
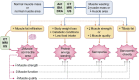
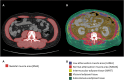
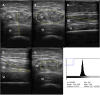
Muscle wasting and low muscle mass are prominent features of protein energy wasting (PEW), sarcopenia and sarcopenic obesity in patients with chronic kidney disease (CKD). In addition, muscle wasting is associated with low muscle strength, impaired muscle function and adverse clinical outcomes such as low quality of life, hospitalizations and increased mortality. While assessment of muscle mass is well justified, the assessment of skeletal muscle should go beyond quantity. Imaging techniques provide the means for non-invasive, comprehensive, in-depth assessment of the quality of the muscle such as the infiltration of ectopic fat. These techniques include computed tomography (CT), magnetic resonance imaging (MRI) and ultrasound. Dual energy X-ray absorptiometry is also an imaging technique, but one that only provides quantitative and not qualitative data on muscle. The main advantage of imaging techniques compared with other methods such as bioelectrical impedance analysis and anthropometry is that they offer higher precision and accuracy. On the other hand, the higher cost for acquiring and maintaining the imaging equipment, especially CT and MRI, makes these less-used options and available mostly for research purposes. In the field of CKD and end-stage kidney disease (ESKD), imaging techniques are gaining attention for evaluating muscle quantity and more recently muscle fat infiltration. This review describes the potential of these techniques in CKD and ESKD settings for muscle assessment beyond that of muscle quantity.
Keywords: chronic kidney disease; computed tomography; magnetic resonance imaging; muscle wasting; ultrasound.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
AS has previously received honoraria and/or paid consultancy from Fresenius Kabi, Dr Schär and Baxter. CMA has previously received honoraria and/or paid consultancy from Fresenius Kabi, Astra-Zeneca and Baxter. BL was supported by a grant from Baxter Healthcare Corporation to Karolinska Institutet. PS has been on scientific advisory boards for Invizius, CSL, Vifor, GSK, Astra Zeneca, Baxter, and Fresenius. PS has lectured at meetings sponsored by Novo Nordisk, Astra Zeneca, Baxter, Astellas, Fresenius and Pfizer. Non of the authors have conflict of interest with the current study.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

