Neuroendocrine effects of the duper mutation in Syrian hamsters: a role for Cryptochrome 1
- PMID: 38444761
- PMCID: PMC10912188
- DOI: 10.3389/fphys.2024.1351682
Neuroendocrine effects of the duper mutation in Syrian hamsters: a role for Cryptochrome 1
Abstract
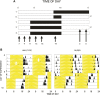
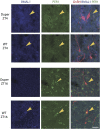
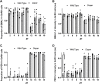
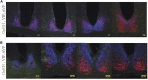
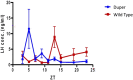
Molecular and physiological determinants of the timing of reproductive events, including the pre-ovulatory LH surge and seasonal fluctuations in fertility, are incompletely understood. We used the Cryptochrome 1-deficient duper mutant to examine the role of this core circadian clock gene in Syrian hamsters. We find that the phase of the LH surge and its stability upon shifts of the light: dark cycle are altered in duper mutants. The intensity of immunoreactive PER1 in GnRH cells of the preoptic area peaks earlier in the day in duper than wild type hamsters. We note that GnRH fibers coursing through the suprachiasmatic nucleus (SCN) contact vasopressin- and VIP-immunoreactive cells, suggesting a possible locus of circadian control of the LH surge. Unlike wild types, duper hamsters do not regress their gonads within 8 weeks of constant darkness, despite evidence of melatonin secretion during the subjective night. In light of the finding that the duper allele is a stop codon in Cryptochrome 1, our results suggest important neuroendocrine functions of this core circadian clock gene.
Keywords: Cryptochrome 1; LH surge; circadian rhythms; duper; melatonin; photoperiod.
Copyright © 2024 Manoogian, Bahiru, Wang, Holder and Bittman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Suprachiasmatic function in a circadian period mutant: Duper alters light-induced activation of vasoactive intestinal peptide cells and PERIOD1 immunostaining.Eur J Neurosci. 2018 Dec;48(11):3319-3334. doi: 10.1111/ejn.14214. Eur J Neurosci. 2018. PMID: 30346078 Free PMC article.
-
Phase resetting in duper hamsters: specificity to photic zeitgebers and circadian phase.J Biol Rhythms. 2015 Apr;30(2):129-43. doi: 10.1177/0748730414568297. Epub 2015 Jan 29. J Biol Rhythms. 2015. PMID: 25633984 Free PMC article.
-
The duper mutation reveals previously unsuspected functions of Cryptochrome 1 in circadian entrainment and heart disease.Proc Natl Acad Sci U S A. 2022 Aug 9;119(32):e2121883119. doi: 10.1073/pnas.2121883119. Epub 2022 Aug 5. Proc Natl Acad Sci U S A. 2022. PMID: 35930669 Free PMC article.
-
Melatonin feedback on clock genes: a theory involving the proteasome.J Pineal Res. 2015 Jan;58(1):1-11. doi: 10.1111/jpi.12189. Epub 2014 Nov 22. J Pineal Res. 2015. PMID: 25369242 Review.
-
Development of the mammalian circadian clock.Eur J Neurosci. 2020 Jan;51(1):182-193. doi: 10.1111/ejn.14318. Epub 2019 Jan 30. Eur J Neurosci. 2020. PMID: 30589961 Review.
References
LinkOut - more resources
Full Text Sources

