Effect of miR-17 on Polygonum Cillinerve polysaccharide against transmissible gastroenteritis virus
- PMID: 38444776
- PMCID: PMC10912159
- DOI: 10.3389/fvets.2024.1360102
Effect of miR-17 on Polygonum Cillinerve polysaccharide against transmissible gastroenteritis virus
Abstract
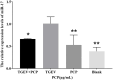
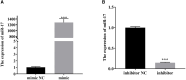
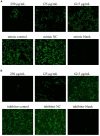
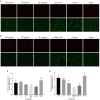
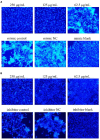
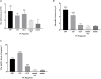
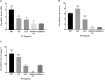
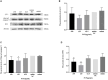
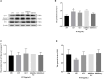
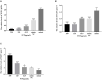
Transmissible gastroenteritis virus (TGEV) could cause diarrhea, vomiting, dehydration and even death in piglets, miRNA played an important role in the interaction between virus and cell. The study aimed to investigate the impact of miR-17 on the polysaccharide of Polygonum Cillinerve (PCP) in combating TGEV. miR-17 was screened and transfection validation was performed by Real-time PCR. The function of miR-17 on PK15 cells infected with TGEV and treated with PCP was investigated by DCFH-DA loading probe, JC-1 staining and Hoechst fluorescence staining. Furthermore, the effect of miR-17 on PCP inhibiting TGEV replication and apoptosis signaling pathways during PCP against TGEV infection was measured through Real-time PCR and Western blot. The results showed that miR-17 mimic and inhibitor could be transferred into PK15 cells and the expression of miR-17 significantly increased and decreased respectively compared with miR-17 mimic and inhibitor (P < 0.05). A total 250 μg/mL of PCP could inhibit cells apoptosis after transfection with miR-17. PCP (250 μg/mL and 125 μg/mL) significantly inhibited the decrease in mitochondrial membrane potential induced by TGEV after transfection with miR-17 (P < 0.05). After transfection of miR-17 mimic, PCP at concentrations of 250 μg/mL and 125 μg/mL significantly promoted the mRNA expression of P53, cyt C and caspase 9 (P < 0.05). Compared with the control group, the replication of TGEV gRNA and gene N was significantly inhibited by PCP at concentrations of 250 μg/mL and 125 μg/mL after transfection of both miR-17 mimic and inhibitor (P < 0.05). PCP at 62.5 μg/mL significantly inhibited the replication of gene S following transfection with miR-17 inhibitor (P < 0.05). These results suggested that PCP could inhibit the replication of TGEV and apoptosis induced by TGEV by regulating miR-17.
Keywords: Polygonum Cillinerve polysaccharide; apoptosis; miR-17; transmissible gastroenteritis virus; virus replication.
Copyright © 2024 Duan, Xu, Wang, Liu, Wang, Liu, Zhang, Ma, Ma and Fan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Polygonum cillinerve polysaccharide inhibits transmissible gastroenteritis virus by regulating microRNA-181.Vet J. 2024 Apr;304:106083. doi: 10.1016/j.tvjl.2024.106083. Epub 2024 Feb 15. Vet J. 2024. PMID: 38365083
-
The inhibitory effect Polygonum Cillinerve polysaccharide on transmissible gastroenteritis virus of swine.Res Vet Sci. 2021 Nov;140:47-55. doi: 10.1016/j.rvsc.2021.08.005. Epub 2021 Aug 8. Res Vet Sci. 2021. PMID: 34399280
-
Study on the immune-enhancing and inhabiting transmissible gastroenteritis virus effects of polysaccharides from Cimicifuga rhizoma.Microb Pathog. 2024 Jul;192:106719. doi: 10.1016/j.micpath.2024.106719. Epub 2024 May 27. Microb Pathog. 2024. PMID: 38810768
-
miR-27b attenuates apoptosis induced by transmissible gastroenteritis virus (TGEV) infection via targeting runt-related transcription factor 1 (RUNX1).PeerJ. 2016 Feb 4;4:e1635. doi: 10.7717/peerj.1635. eCollection 2016. PeerJ. 2016. PMID: 26870610 Free PMC article.
-
Transmissible Gastroenteritis Virus: An Update Review and Perspective.Viruses. 2023 Jan 27;15(2):359. doi: 10.3390/v15020359. Viruses. 2023. PMID: 36851573 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

