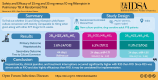
Safety and Efficacy of 25 mg/kg and 35 mg/kg vs 10 mg/kg Rifampicin in Pulmonary TB: A Phase IIb Randomized Controlled Trial
- PMID: 38444824
- PMCID: PMC10914527
- DOI: 10.1093/ofid/ofae034
Safety and Efficacy of 25 mg/kg and 35 mg/kg vs 10 mg/kg Rifampicin in Pulmonary TB: A Phase IIb Randomized Controlled Trial
Abstract
Background: Globally, no trial data are available on head-to-head comparison between 10 mg/kg and 25/35 mg/kg rifampicin in treating pulmonary tuberculosis during study initiation.
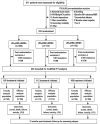
Methods: A multicentric, phase IIb randomized trial recruited 333 new culture-positive, drug-sensitive adult patients with pulmonary tuberculosis to compare safety and efficacy of high-dose rifampicin (R25/R35), against conventional dose (R10) given daily for 8 weeks followed by standard doses for 16 weeks. Main outcomes were treatment-emergent grade 3/4 adverse events (AEs) and time-to-culture conversion in liquid media, assessed by division of AIDS system for grading the severity of adverse events division of AIDS criteria and Kaplan-Meier methods.
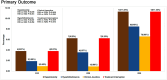
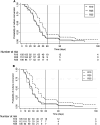
Results: In a modified intention-to-treat population of 323 patients (R10: 105/R25: 112/R35: 106), grade 3/4 AEs were reported in 34 patients (R10: 9.5% [10/105], R25: 9.8% [11/112], R35: 12.3% [13/106]) during the intensive phase. Among 23 patients (R10: 3.8% [4/105], R25: 6.3% [7/112], R35: 11.3% [12/106]) with grade 3/4 hepatotoxicity, 15 (R10: 1.9% [2/105], R25: 3.6% [4/112], R35: 8.5% [9/106]) had grade 3/4 hyperbilirubinemia and 9 patients (R10: 1.0% [1/105], R25: 0.9% [1/112], R35: 6.6% [7/106]) developed clinical jaundice. Significant differences observed only between R10 and R35 with hepatotoxicity (P = .039), hyperbilirubinemia (P = .031), clinical jaundice (P = .032), and treatment interruption (P = .039). Eighteen serious AEs and 6 deaths (R10: 3/R25: 1/R35: 2) occurred during study period. Time to stable culture conversion in liquid media was faster in R25 (adjusted hazard ratio, 1.71; 95% confidence interval [CI], 1.26-2.31 [solid: 1.97; 95% CI, 1.46-2.67]) and R35 (1.81; 95% CI, 1.33-2.48 [solid: 2.24; 95% CI, 1.64-3.06]), than R10 (34 vs 44 days). R25 had no failure/relapse.
Conclusions: Hepatotoxicity, clinical jaundice, and treatment interruptions occurred significantly higher with R35 than R10. Because R25 was comparably safe as R10 and also highly efficacious than R10, it may be considered for implementation. Clinical Trials Registration. CTRI/2017/12/010951.
Keywords: efficacy; high-dose; rifampicin; safety; time-to-culture-conversion.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts.
Figures
Similar articles
-
High-Dose Rifampicin Mediated Systemic Alterations of Cytokines, Chemokines, Growth Factors, Microbial Translocation Markers, and Acute-Phase Proteins in Pulmonary Tuberculosis.Front Pharmacol. 2022 Jul 15;13:896551. doi: 10.3389/fphar.2022.896551. eCollection 2022. Front Pharmacol. 2022. PMID: 35910352 Free PMC article.
-
High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial.Lancet Infect Dis. 2017 Jan;17(1):39-49. doi: 10.1016/S1473-3099(16)30274-2. Epub 2016 Oct 26. Lancet Infect Dis. 2017. PMID: 28100438 Free PMC article. Clinical Trial.
-
Bedaquiline-pretomanid-moxifloxacin-pyrazinamide for drug-sensitive and drug-resistant pulmonary tuberculosis treatment: a phase 2c, open-label, multicentre, partially randomised controlled trial.Lancet Infect Dis. 2024 Sep;24(9):1003-1014. doi: 10.1016/S1473-3099(24)00223-8. Epub 2024 May 17. Lancet Infect Dis. 2024. PMID: 38768617 Clinical Trial.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Shortened treatment regimens versus the standard regimen for drug-sensitive pulmonary tuberculosis.Cochrane Database Syst Rev. 2019 Dec 12;12(12):CD012918. doi: 10.1002/14651858.CD012918.pub2. Cochrane Database Syst Rev. 2019. PMID: 31828771 Free PMC article.
Cited by
-
Exposure to Rifampicin and its Metabolite 25-Deacetylrifampicin Rapidly Decreases During Tuberculosis Therapy.Clin Pharmacokinet. 2025 Mar;64(3):387-396. doi: 10.1007/s40262-025-01479-3. Epub 2025 Jan 27. Clin Pharmacokinet. 2025. PMID: 39871048 Free PMC article.
-
Efficacy and safety of higher dose rifampicin in adults with presumed drug-susceptible tuberculosis: an updated systematic review and meta-analysis.EClinicalMedicine. 2024 Oct 3;77:102857. doi: 10.1016/j.eclinm.2024.102857. eCollection 2024 Nov. EClinicalMedicine. 2024. PMID: 39416385 Free PMC article.
-
Strategies for shortening tuberculosis therapy.Nat Med. 2025 Jun;31(6):1765-1775. doi: 10.1038/s41591-025-03742-3. Epub 2025 Jun 13. Nat Med. 2025. PMID: 40514466 Free PMC article. Review.
-
Safety of Triple-Dose Rifampin in Tuberculosis Treatment: A Systematic Review and Meta-Analysis.Clin Infect Dis. 2025 Aug 1;81(1):119-128. doi: 10.1093/cid/ciaf004. Clin Infect Dis. 2025. PMID: 39789804 Free PMC article.
References
-
- Thamineni R, Peraman R, Chenniah J. Level of adherence to anti-tubercular treatment among drug-sensitive tuberculosis participants on a newly introduced daily dose regimen in South India: a cross-sectional study. Trop Med Int Health 2022; 27:1013–23. - PubMed
-
- Jindani A, Atwine D, Grint D, et al. Four-month high-dose rifampicin regimens for pulmonary tuberculosis. NEJM Evid 2023; 2:9. doi:10.1056/EVIDoa2300054 https://evidence.nejm.org/doi/abs/10.1056/EVIDoa2300054 - DOI - DOI - PubMed

