Tick cysteine protease inhibitors suppress immune responses in mannan-induced psoriasis-like inflammation
- PMID: 38444844
- PMCID: PMC10912570
- DOI: 10.3389/fimmu.2024.1344878
Tick cysteine protease inhibitors suppress immune responses in mannan-induced psoriasis-like inflammation
Abstract
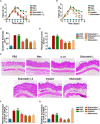
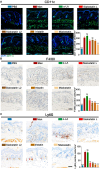
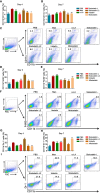
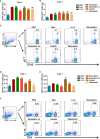
Protease inhibitors regulate various biological processes and prevent host tissue/organ damage. Specific inhibition/regulation of proteases is clinically valuable for treating several diseases. Psoriasis affects the skin in the limbs and scalp of the body, and the contribution of cysteine and serine proteases to the development of skin inflammation is well documented. Cysteine protease inhibitors from ticks have high specificity, selectivity, and affinity to their target proteases and are efficient immunomodulators. However, their potential therapeutic effect on psoriasis pathogenesis remains to be determined. Therefore, we tested four tick cystatins (Sialostatin L, Sialostatin L2, Iristatin, and Mialostatin) in the recently developed, innate immunity-dependent mannan-induced psoriasis model. We explored the effects of protease inhibitors on clinical symptoms and histological features. In addition, the number and percentage of immune cells (dendritic cells, neutrophils, macrophages, and γδT cells) by flow cytometry, immunofluorescence/immunohistochemistry and, the expression of pro-inflammatory cytokines (TNF-a, IL-6, IL-22, IL-23, and IL-17 family) by qPCR were analyzed using skin, spleen, and lymph node samples. Tick protease inhibitors have significantly decreased psoriasis symptoms and disease manifestations but had differential effects on inflammatory responses and immune cell populations, suggesting different modes of action of these inhibitors on psoriasis-like inflammation. Thus, our study demonstrates, for the first time, the usefulness of tick-derived protease inhibitors for treating skin inflammation in patients.
Keywords: autoimmune disease; immune responses; protease inhibitors; psoriasis; tick.
Copyright © 2024 Wu, Jmel, Chai, Tian, Xu, Hui, Nandakumar and Kotsyfakis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were editorial board members of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
The role of cystatins in tick physiology and blood feeding.Ticks Tick Borne Dis. 2012 Jun;3(3):117-27. doi: 10.1016/j.ttbdis.2012.03.004. Epub 2012 Apr 20. Ticks Tick Borne Dis. 2012. PMID: 22647711 Free PMC article. Review.
-
Comparative studies on mannan and imiquimod induced experimental plaque psoriasis inflammation in inbred mice.Clin Exp Immunol. 2023 Mar 24;211(3):288-300. doi: 10.1093/cei/uxad004. Clin Exp Immunol. 2023. PMID: 36645209 Free PMC article.
-
Estrogen Acts Through Estrogen Receptor-β to Promote Mannan-Induced Psoriasis-Like Skin Inflammation.Front Immunol. 2022 May 19;13:818173. doi: 10.3389/fimmu.2022.818173. eCollection 2022. Front Immunol. 2022. PMID: 35663991 Free PMC article.
-
Bruton's tyrosine kinase inhibitor suppresses imiquimod-induced psoriasis-like inflammation in mice through regulation of IL-23/IL-17A in innate immune cells.Int Immunopharmacol. 2020 Mar;80:106215. doi: 10.1016/j.intimp.2020.106215. Epub 2020 Jan 24. Int Immunopharmacol. 2020. PMID: 31982823
-
Proteases and protease inhibitors in saliva of hard ticks: Biological role and pharmacological potential.Adv Parasitol. 2024;126:229-251. doi: 10.1016/bs.apar.2024.09.001. Epub 2024 Oct 18. Adv Parasitol. 2024. PMID: 39448192 Review.
Cited by
-
Unveiling ferroptosis: a new frontier in skin disease research.Front Immunol. 2024 Oct 4;15:1485523. doi: 10.3389/fimmu.2024.1485523. eCollection 2024. Front Immunol. 2024. PMID: 39430757 Free PMC article. Review.
-
The Therapeutic Potential of Kiwi Extract as a Source of Cysteine Protease Inhibitors on DNCB-Induced Atopic Dermatitis in Mice and Human Keratinocyte HaCaT Cells.Int J Mol Sci. 2025 Feb 12;26(4):1534. doi: 10.3390/ijms26041534. Int J Mol Sci. 2025. PMID: 40004009 Free PMC article.
-
Therapeutic Effect of Lecigel, Cetiol®CC, Activonol-6, Activonol-M, 1,3-Propanediol, Soline, and Fucocert® (LCAA-PSF) Treatment on Imiquimod-Induced Psoriasis-like Skin in Mice.Int J Mol Sci. 2024 Jul 14;25(14):7720. doi: 10.3390/ijms25147720. Int J Mol Sci. 2024. PMID: 39062965 Free PMC article.
-
Ticks' tricks: immunomodulatory effects of ixodid tick saliva at the cutaneous tick-host interface.Front Immunol. 2025 Mar 27;16:1520665. doi: 10.3389/fimmu.2025.1520665. eCollection 2025. Front Immunol. 2025. PMID: 40213541 Free PMC article. Review.
-
Amblyostatin-1, the first salivary cystatin with host immunomodulatory and anti-inflammatory properties from the Neotropical tick Amblyomma sculptum, vector of Brazilian spotted fever.Front Immunol. 2025 Jul 17;16:1585703. doi: 10.3389/fimmu.2025.1585703. eCollection 2025. Front Immunol. 2025. PMID: 40746554 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

