Oral administration of Manuka honey induces IFNγ-dependent resistance to tumor growth that correlates with beneficial modulation of gut microbiota composition
- PMID: 38444857
- PMCID: PMC10912506
- DOI: 10.3389/fimmu.2024.1354297
Oral administration of Manuka honey induces IFNγ-dependent resistance to tumor growth that correlates with beneficial modulation of gut microbiota composition
Abstract
Background: To investigate the potential of Manuka honey (MH) as an immunomodulatory agent in colorectal cancer (CRC) and dissect the underlying molecular and cellular mechanisms.
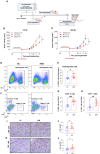
Methods: MH was administered orally over a 4 week-period. The effect of MH treatment on microbiota composition was studied using 16S rRNA sequencing of fecal pellets collected before and after treatment. Pretreated mice were implanted with CRC cells and followed for tumor growth. Tumors and lymphoid organs were analyzed by flow cytometry (FACS), immunohistochemistry and qRT-PCR. Efficacy of MH was also assessed in a therapeutic setting, with oral treatment initiated after tumor implantation. We utilized IFNγ-deficient mice to determine the importance of interferon signaling in MH-induced immunomodulation.
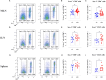
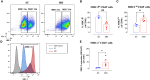
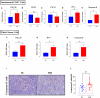
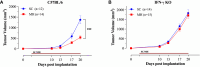
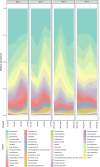
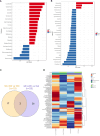
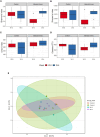
Results: Pretreatment with MH enhanced anti-tumor responses leading to suppression of tumor growth. Evidence for enhanced tumor immunogenicity included upregulated MHC class-II on intratumoral macrophages, enhanced MHC class-I expression on tumor cells and increased infiltration of effector T cells into the tumor microenvironment. Importantly, oral MH was also effective in retarding tumor growth when given therapeutically. Transcriptomic analysis of tumor tissue highlighted changes in the expression of various chemokines and inflammatory cytokines that drive the observed changes in tumor immunogenicity. The immunomodulatory capacity of MH was abrogated in IFNγ-deficient mice. Finally, bacterial 16S rRNA sequencing demonstrated that oral MH treatment induced unique changes in gut microbiota that may well underlie the IFN-dependent enhancement in tumor immunogenicity.
Conclusion: Our findings highlight the immunostimulatory properties of MH and demonstrate its potential utilization in cancer prevention and treatment.
Keywords: Manuka honey; colorectal cancer; immunomodulation; tumor immunogenicity; type I/II IFN.
Copyright © 2024 Masad, Idriss, Mohamed, Al-Sbiei, Bashir, Al-Marzooq, Altahrawi, Fernandez-Cabezudo and Al-Ramadi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Characterization of immunomodulatory responses induced by manuka honey.Front Immunol. 2022 Nov 2;13:1020574. doi: 10.3389/fimmu.2022.1020574. eCollection 2022. Front Immunol. 2022. PMID: 36405698 Free PMC article.
-
Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice humanized with gut microbiota from patients with colorectal cancer.Theranostics. 2021 Feb 19;11(9):4155-4170. doi: 10.7150/thno.54476. eCollection 2021. Theranostics. 2021. PMID: 33754054 Free PMC article.
-
Intratumoral Microbiota Changes with Tumor Stage and Influences the Immune Signature of Oral Squamous Cell Carcinoma.Microbiol Spectr. 2023 Aug 17;11(4):e0459622. doi: 10.1128/spectrum.04596-22. Epub 2023 Jul 6. Microbiol Spectr. 2023. PMID: 37409975 Free PMC article.
-
The super-food Manuka honey, a comprehensive review of its analysis and authenticity approaches.J Food Sci Technol. 2022 Jul;59(7):2527-2534. doi: 10.1007/s13197-021-05181-7. Epub 2021 Jun 30. J Food Sci Technol. 2022. PMID: 35734106 Free PMC article. Review.
-
An updated review of functional ingredients of Manuka honey and their value-added innovations.Food Chem. 2024 May 15;440:138060. doi: 10.1016/j.foodchem.2023.138060. Epub 2023 Nov 24. Food Chem. 2024. PMID: 38211407 Review.
Cited by
-
Antibacterial Activity and Prebiotic Properties of Six Types of Lamiaceae Honey.Antibiotics (Basel). 2024 Sep 10;13(9):868. doi: 10.3390/antibiotics13090868. Antibiotics (Basel). 2024. PMID: 39335041 Free PMC article.
-
Manuka honey as a non-antibiotic alternative against Staphylococcus spp. and their small colony variant (SCVs) phenotypes.Front Cell Infect Microbiol. 2024 May 28;14:1380289. doi: 10.3389/fcimb.2024.1380289. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38868298 Free PMC article. Review.
-
Antibiotics-induced dysbiosis impacts dendritic morphology of adult mouse cortical interneurons.Front Neuroanat. 2025 Mar 7;19:1557961. doi: 10.3389/fnana.2025.1557961. eCollection 2025. Front Neuroanat. 2025. PMID: 40124111 Free PMC article.
-
Impact of Mānuka Honey on Symptoms and Quality of Life in Individuals With Functional Dyspepsia: Protocol for a Feasibility Randomized Controlled Trial.JMIR Res Protoc. 2025 May 21;14:e66417. doi: 10.2196/66417. JMIR Res Protoc. 2025. PMID: 40397937 Free PMC article.
References
-
- WHO . Cancer(2022). Available online at: https://www.who.int/news-room/fact-sheets/detail/cancer.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

