Presentation and progression of MPO-ANCA interstitial lung disease
- PMID: 38445024
- PMCID: PMC10912625
- DOI: 10.1016/j.jtauto.2024.100235
Presentation and progression of MPO-ANCA interstitial lung disease
Abstract
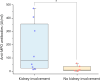
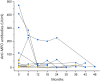
The association between MPO-ANCA-associated vasculitis (AAV) and interstitial lung disease (ILD) has been well established. Pulmonary fibrosis may coexist with, follow, or even precede the diagnosis of AAV, and its presence adversely affects the prognosis. The optimal approach to investigating ANCA in patients with ILD remains a subject of ongoing debate. Here we aim to describe presentation and progression of MPO-ANCA ILD. We conducted a retrospective evaluation of a cohort of individuals diagnosed with MPO-ANCA ILD, with or without accompanying renal impairment, at the Immunology and Cell Therapy Unit, Careggi University Hospital, Florence, Italy, between June 2016 and June 2022. Clinical records, imaging studies, pathologic examinations, and laboratory test results were collected. Among the 14 patients identified with MPO-ANCA ILD, we observed a significant association between MPO-ANCA titers assessed at the time of ILD diagnosis and renal involvement. Renal impairment in these cases often manifested as subclinical or slowly progressive kidney damage. Interestingly, complement C3 deposits were consistently found in all renal biopsy specimens, thereby suggesting the potential for novel therapeutic targets in managing renal complications associated with MPO-ANCA ILD. The presentation of MPO-ANCA vasculitis as ILD can be the first and only clinical manifestation. MPO-ANCA levels at ILD diagnosis could warn on the progression to renal involvement in patients with MPO-ANCA ILD, hence caution is needed because renal disease can be subclinical or smoldering.
Keywords: ANCA-Associated vasculitis; C3 deposits; Glomerulonephritis; Interstitial lung disease; MPO; MPO-ANCA; Pulmonary fibrosis; UIP.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Interstitial lung disease in patients with antineutrophil cytoplasmic antibody-associated vasculitis: chest CT patterns and correlation with survival.Semin Arthritis Rheum. 2025 Aug;73:152726. doi: 10.1016/j.semarthrit.2025.152726. Epub 2025 Apr 10. Semin Arthritis Rheum. 2025. PMID: 40273744
-
Usual interstitial pneumonia in ANCA-associated vasculitis: A poor prognostic factor.J Autoimmun. 2020 Jan;106:102338. doi: 10.1016/j.jaut.2019.102338. Epub 2019 Sep 27. J Autoimmun. 2020. PMID: 31570253
-
Radiologic and pathologic characteristics of myeloperoxidase-antineutrophil cytoplasmic antibody-associated interstitial lung disease: a retrospective analysis.Sarcoidosis Vasc Diffuse Lung Dis. 2019;36(3):195-201. doi: 10.36141/svdld.v36i3.8053. Epub 2019 May 1. Sarcoidosis Vasc Diffuse Lung Dis. 2019. PMID: 32476954 Free PMC article.
-
Acute interstitial nephritis caused by ANCA-associated vasculitis: a case based review.Clin Rheumatol. 2024 Mar;43(3):1227-1244. doi: 10.1007/s10067-023-06798-z. Epub 2023 Nov 6. Clin Rheumatol. 2024. PMID: 37932622 Review.
-
Anti-Inflammatory and/or Anti-Fibrotic Treatment of MPO-ANCA-Positive Interstitial Lung Disease: A Short Review.J Clin Med. 2022 Jul 1;11(13):3835. doi: 10.3390/jcm11133835. J Clin Med. 2022. PMID: 35807120 Free PMC article. Review.
Cited by
-
Clinical Insights and Therapeutic Strategies for the Treatment of Interstitial Lung Disease in Patients with Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis: Current Trends and Future Directions.J Clin Med. 2025 Jun 30;14(13):4631. doi: 10.3390/jcm14134631. J Clin Med. 2025. PMID: 40649005 Free PMC article. Review.
-
Current Diagnosis and Treatment of Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis: A Review Including a Comparison of Characteristics in Europe and Japan.J Clin Med. 2025 Mar 4;14(5):1724. doi: 10.3390/jcm14051724. J Clin Med. 2025. PMID: 40095851 Free PMC article. Review.
-
Distinct pulmonary patterns in ANCA-associated vasculitides: insights from a retrospective single center cohort study.Rheumatol Int. 2024 Nov;44(11):2435-2443. doi: 10.1007/s00296-024-05664-8. Epub 2024 Aug 13. Rheumatol Int. 2024. PMID: 39136785
References
-
- Jennette J.C., Falk R.J., Bacon P.A., Basu N., Cid M.C., Ferrario F., Flores-Suarez L.F., Gross W.L., Guillevin L., Hagen E.C., Hoff-man G.S., Jayne D.R., Kallenberg C.G., Lamprecht P., Langford C.A., Luqmani R.A., Mahr A.D., Matteson E.L., Merkel P.A., Ozen S., Pusey C.D., Rasmussen N., Rees A.J., Scott D.G., Specks U., Stone J.H., Takahashi K., Watts R.A. Revised international Chapel Hill consensus Conference Nomenclature of vasculitides. Arthritis Rheum. 2012;65(1):1–11. doi: 10.1002/art.37715. 2013 Jan. - DOI - PubMed
-
- Lyons P.A., Peters J.E., Alberici F., Liley J., Coulson R.M.R., Astle W., Baldini C., Bonatti F., Cid M.C., Elding H., Emmi G., Epplen J., Guillevin L., Jayne D.R.W., Jiang T., Gunnarsson I., Lamprecht P., Leslie S., Little M.A., Martorana D., Moosig F., Neumann T., Ohlsson S., Quickert S., Ramirez G.A., Rewerska B., Schett G., Sinico R.A., Szczeklik W., Tesar V., Vukcevic D., European Vas-culitis Genetics Consortium, Terrier B., Watts R.A., Vaglio A., Ju Holle, Wallace C., Smith K.G.C. Genome-wide association study of eosinophilic granulomatosis with polyangiitis reveals genomic loci stratified by ANCA status. Nat. Commun. 2019 Nov 12;10(1):5120. doi: 10.1038/s41467-019-12515-9. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

